ICES-2021-292.Pdf (1.890Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
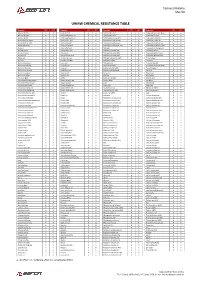
Chemical Properties
Technical Bulletin Mar/20 UHMW CHEMICAL RESISTANCE TABLE Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Acetic acid 10% A A Cetyl alcohol A A Hydrobromic acid A A Potassium ferrocyanide sat. A A Acetic acid 100% A B Chlorinated water 2% A A Hydrobromic acid B X Potassium fluoride A A Acetic acid 60% A A Chlorinated water sat. A B Hydrobromic acid aq.50% A A Potassium hydroxide A A Acetic aldehyde 100% B X Chlorine (dry gas) B X Hydrochloric acid aq.10% A A Potassium nitrate sat. A A Acetic aldehyde 40% B X Chlorine (liquid) B X Hydrocyanic acid aq.sat. A A Potassium perborate sat. A A Acetic anhydride A B Chlorine (wet gas) B X Hydrofluoric acid aq.40-75% A A Potassium perchlorate 10% A A Acetone A A ChlorineBenzene B X Hydrogen A A Potassium permanganate A A Acetophenone B A Chloroacetic acid X X Hydrogen bromide 10% A A Potassium sulfite A A Acrylic emulsion A A Chloroform X X Hydrogen peroxide 30% A A Potassium sulphate conc. A A Acrylonitrile A A Chlorosulfonic acid X X Hydrogen peroxide 90% A B Potassium sulphide conc. A A Adipic acid A A Chrome alum sat. A A Hydrogen phosphite 100% A A Propane (gas) A A Alumens A A Chromic acid 80% A A Hydrogen sulfide A A Propanol A A Aluminum acetate A A Citric acid A A Hydroquinone A A Propargyl alcohol A A Aluminum chloride A A Citronella oil B X Iodine (in alcohol) B B Propylene dichloride 100% X X Aluminum fluoride A A Clove oil A B Isobutyl alcohol 100% A A Propylene glycol A A Aluminum hydroxide A A Coclohexanona B X Isopropyl alcohol 100% A A Pyridine A B Aluminum oxalate A A Coconut oil A A Kerosene A B Resorcinol A A Aluminum sulfate A A Cod liver oil A A Ketchup A A Royal water B B Ammonia (gas) A A Coffee A A Lactic acid 10-90% A A Salicylic acid A A Ammoniacal ferrous citrate A A Copper chloride sat. -

APP202482 APP202482 Application Form Final.Pdf(PDF, 920
Application for the modified reassessment of a hazardous substance Under Section 63A of the Hazardous Substances and New Organisms Act 1996 Chemical Review 2012 – 2014 A modified reassessment of a range of substances for which new information was obtained in the period 2012 - 2014 Application number: APP202482 Applicant: Chief Executive, Environmental Protection Authority www.epa.govt.nz Chemical Review 2012 – 2014 (APP202482) 2 Applicant’s details Name: Rob Forlong, Chief Executive Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5403 Fax: 04 914 0433 Email: [email protected] Applicant’s contact person Name: Asela Atapattu Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5463 Fax: 04 914 0433 Email: [email protected] Signature of Applicant 3 June 2015 Rob Forlong Date Chief Executive Environmental Protection Authority Chemical Review 2012 – 2014 (APP202482) 3 Background The Environmental Protection Authority regularly receives new information from stakeholders regarding the classifications and controls of substances. EPA staff also note where changes to approvals are needed. Where those changes are not minor or technical, these changes require a reassessment or a modified reassessment of the approval of the substance under the HSNO Act 1996 (“the Act”) The Chemical Review is intended as a means of making changes to a number of approvals at once, taking into account the new information available to the EPA. This is undertaken as a modified reassessment under section 63A of the Act. This application makes recommendations to change some or all of the following aspects of the approvals in this application: - The approval name of the substance - The hazard classification(s) applied to the substance - The controls applied to the substance The controls changes proposed are largely as a result of changes to the hazard classifications of the substances in this application. -

Water Loss from Terrestrial Planets with CO2-Rich Atmospheres
Water loss from terrestrial planets with CO2-rich atmospheres R. D. Wordsworth Department of the Geophysical Sciences, University of Chicago, 60637 IL, USA [email protected] and R. T. Pierrehumbert Department of the Geophysical Sciences, University of Chicago, 60637 IL, USA ABSTRACT Water photolysis and hydrogen loss from the upper atmospheres of terrestrial planets is of fundamental importance to climate evolution but remains poorly understood in general. Here we present a range of calculations we performed to study the dependence of water loss rates from terrestrial planets on a range of atmospheric and external parameters. We show that CO2 can only cause sig- nificant water loss by increasing surface temperatures over a narrow range of conditions, with cooling of the middle and upper atmosphere acting as a bottle- neck on escape in other circumstances. Around G-stars, efficient loss only occurs on planets with intermediate CO2 atmospheric partial pressures (0.1 to 1 bar) that receive a net flux close to the critical runaway greenhouse limit. Because G-star total luminosity increases with time but XUV/UV luminosity decreases, this places strong limits on water loss for planets like Earth. In contrast, for a CO2-rich early Venus, diffusion limits on water loss are only important if clouds caused strong cooling, implying that scenarios where the planet never had surface liquid water are indeed plausible. Around M-stars, water loss is primarily a func- arXiv:1306.3266v2 [astro-ph.EP] 12 Oct 2013 tion of orbital distance, with planets that absorb less flux than ∼ 270 W m−2 (global mean) unlikely to lose more than one Earth ocean of H2O over their lifetimes unless they lose all their atmospheric N2/CO2 early on. -
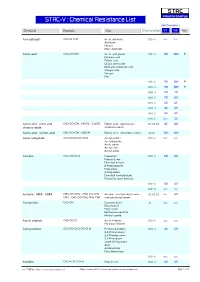
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Cold Traps out of Glass Or Stainless Steel for the Vacuum Technology
Cold traps out of glass or stainless steel for the vacuum technology KGW-ISOTHERM Karlsruher Glastechnisches Werk 76185 Karlsruhe Gablonzerstraße 6 Tel:0721/ 95897-0 Fax: 0721 / 95897-77 Email: [email protected] Internet: www.kgw-isotherm.com A 16 Cold traps: construction, operation and principles Cold traps are used in conjunction with vacuum pumps to collect condensation produced from humidity or solvents and these cold traps can be used for many different tasks. The most common application is collecting condensation produced from humidity or solvents from rotating discs, vacuum pumps or high vacuum systems that use‘s oil diffusion or turbo-molecular pumps. In this case a common coolant such as liquid nitrogen (LN2) or dry-ice (CO2) with acetone is normally used. Another application is the production of condensation from specific substances at a constant, predefined temperature. This can be realised by using a coolant at a constant, predefined temperature, a thermostat or a Kaltgas system. Cold traps can be manufactured out of glass or metal. The use of glass is advantageous in the chemical sector and when producing condensation from solvents, due to its resistance to chemicals. All glass cold traps listed in this catalogue are produced solely from borosilicate glass 3.3, in compliance with DIN/ISO (DURAN made by Schott). The mechanical design takes into account the wall thickness for use under vacuum. Material - glass All the glassware produced by KGW - ISOTHERM are made of borosilicat glass 3.3 DIN/ISO 3585. The glass has the following characteristics: Chemical characteristics hydrolytic resistance : according to DIN-ISO 719 (98°C) acid resistance : according to DIN-ISO 1776 alkaline resistance : according to ISO 695-A2 Physical characteristics linear expansion factor : 3,3 x 10-6 1/K (at 20°C-300°C) density : 2,23 g/cm3 specific thermal capacity : 910 J/kg K transformation temperature : 525 °C Admissible Operation Conditions for cold traps made of glass Temperature range -200°C to +200 °C Pressure range standard vacuum to atm. -

Environmental Health and Safety Vacuum Traps
Environmental Health and Safety Vacuum Traps Always place an appropriate trap between experimental apparatus and the vacuum source. The vacuum trap: • protects the pump, pump oil and piping from the potentially damaging effects of the material; • protects people who must work on the vacuum lines or system, and; • prevents vapors and related odors from being emitted back into the laboratory or system exhaust. Improper trapping can allow vapor to be emitted from the exhaust of the vacuum system, resulting in either reentry into the laboratory and building or potential exposure to maintenance workers. Proper traps are important for both local pumps and building systems. Proper Trapping Techniques To prevent contamination, all lines leading from experimental apparatus to the vacuum source must be equipped with filtration or other trapping as appropriate. • Particulates: use filtration capable of efficiently trapping the particles in the size range being generated. • Biological Material: use a High Efficiency Particulate Air (HEPA) filter. Liquid disinfectant (e.g. bleach or other appropriate material) traps may also be required. • Aqueous or non-volatile liquids: a filter flask at room temperature is adequate to prevent liquids from getting to the vacuum source. • Solvents and other volatile liquids: use a cold trap of sufficient size and cold enough to condense vapors generated, followed by a filter flask capable of collecting fluid that could be aspirated out of the cold trap. • Highly reactive, corrosive or toxic gases: use a sorbent canister or scrubbing device capable of trapping the gas. Environmental Health and Safety 632-6410 January 2010 EHSD0365 (01/10) Page 1 of 2 www.stonybrook.edu/ehs Cold Traps For most volatile liquids, a cold trap using a slush of dry ice and either isopropanol or ethanol is sufficient (to -78 deg. -

Hearing Conservation
Stench Chemicals Stench chemicals are a group of chemicals that exhibit an extremely foul smell. Even though most stench chemicals have little direct impact on the physical health and safety of researchers, use of these chemicals without an odor control plan can have an effect on both the laboratory environment as well as the outside environment. Therefore, it is important that researchers carefully control their use of all stench chemicals in order to minimize the impact on other workers and neighbors. Examples of Stench Compounds A few examples of stench chemicals are: thiols (mercaptans), sulfides, selenides, amines, phosphines, butyric acid and valeric acid. Among the chemicals listed above, thiols, also referred to as mercaptans, are the most commonly used group, and are therefore discussed in further detail below. Thiols (Mercaptans) Thiols are a class of organosulfur compounds characterized by the presence of one or more sulfhydryl (-SH) groups. Since 1937, thiols have been added to natural gas (which is naturally odorless) as an odorant to assist with detecting natural gas leaks. Thiols exhibit a moderate level of toxicity, and their use does not require special safety precautions. Thiols are characterized by their disagreeable odors at parts per million (ppm) concentrations, and have an odor threshold of only 0.011 ppm. Due to their addition to natural gas, the detection of their odor automatically leads to suspicion of natural gas leaks in or near buildings where they are used. Thus, the use of even small quantities of thiols in laboratory experiments without odor control measures in place can cause concern for other building occupants, people passing by outside, or even the occupants of adjacent buildings. -

Sowers NIAC Final Report
Thermal Mining of Ices on Cold Solar System Bodies NIAC Phase I Final Report February 2019 George Sowers 1 Purpose This is the final report of the NASA Innovative Advanced Concepts (NIAC) Phase I study: Thermal Mining of Ices on Cold Solar System Bodies. It is submitted as partial fulfillment of the obligations of the Colorado School of Mines (CSM) under grant number 80NSSC19K0964. 2 Table of Contents List of Figures 5 List of Tables 9 1.0 Executive Summary 10 2.0 Introduction 15 3.0 Solar System Survey of Thermal Mining Targets 18 3.1 Potential Thermal Mining Targets 19 3.2 Thermal Mining Beyond the Moon 32 4.0 Thermal Mining Mission Context: Lunar Polar Ice Mining 34 4.1 Lunar Polar Ice Distribution Analysis 36 4.2 System Architecture 39 4.3 Functional Analysis 42 4.4 Ice Extraction Subsystem 46 4.5 Power Subsystem 53 4.6 Deployment and Setup 55 4.7 Operations 59 4.8 Mass and Cost Estimates 66 4.8.1 Subsystem Mass Estimates 66 4.8.2 Total Mass 70 4.8.3 Subsystem Cost Estimates 71 4.8.4 Total Cost 74 4.9 Business Case Analysis 76 4.9.1 The Propellant Market 76 4.9.2 Business Case Scenarios 80 4.9.3 Business Case Results 83 4.9.4 Comparison to Previous Analysis 87 5.0 Proof of Concept Testing 91 5.1 Testing Objectives and Approach 91 5.2 Icy Regolith Simulants 91 5.3 Block 1 Testing 95 5.3.1 Block 1 Apparatus 95 5.3.2 Block 1 Methodology 96 5.3.3 Block 1 Results 97 5.4 Block 2 Testing 105 5.4.1 Block 2 Apparatus 105 5.4.2 Block 2 Methodology 105 5.4.3 Block 2 Results 105 5.5 Test Conclusions 106 6.0 Summary and Conclusions 115 6.1 Bulletized Summary 115 6.2 Conclusions 116 6.3 Recommendations for Future Work 118 7.0 References 121 8.0 Appendix A: Solar System Catalogue 129 9.0 Appendix B: Acronym List 132 3 Acknowledgements This report was prepared by George Sowers, Ross Centers, David Dickson, Adam Hugo, Curtis Purrington, and Elizabeth Scott. -

Development and Analysis of Cold Trap for Use in Fission Surface Power-Primary Test Circuit
National Aeronautics and NASA/TP—2012–217453 Space Administration IS20 George C. Marshall Space Flight Center Huntsville, Alabama 35812 Development and Analysis of Cold Trap for Use in Fission Surface Power-Primary Test Circuit T.M. Wolfe Department of the Navy, Naval Sea Systems Command, Washington, DC C.A. Dervan Georgia Institute of Technology, Atlanta, Georgia J.B. Pearson and T.J. Godfroy Marshall Space Flight Center, Huntsville, Alabama January 2012 The NASA STI Program…in Profile Since its founding, NASA has been dedicated to the • CONFERENCE PUBLICATION. Collected advancement of aeronautics and space science. The papers from scientific and technical conferences, NASA Scientific and Technical Information (STI) symposia, seminars, or other meetings sponsored Program Office plays a key part in helping NASA or cosponsored by NASA. maintain this important role. • SPECIAL PUBLICATION. Scientific, technical, The NASA STI Program Office is operated by or historical information from NASA programs, Langley Research Center, the lead center for projects, and mission, often concerned with NASA’s scientific and technical information. The subjects having substantial public interest. NASA STI Program Office provides access to the NASA STI Database, the largest collection of • TECHNICAL TRANSLATION. aeronautical and space science STI in the world. English-language translations of foreign The Program Office is also NASA’s institutional scientific and technical material pertinent to mechanism for disseminating the results of its NASA’s mission. research and development activities. These results are published by NASA in the NASA STI Report Specialized services that complement the STI Series, which includes the following report types: Program Office’s diverse offerings include creating custom thesauri, building customized databases, • TECHNICAL PUBLICATION. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Water Circulation and Cold Trap
Summary Water Circulator (Chiller) & Cold Trap CF301 CF800 CA301 CA801 Water Circulator (Chiller) Cold Trap Water Circulator (Chiller) Cold Trap Purpose Purpose Supplies a source of temperature Efficiently collects moisture and harmful vapors by trapping them in the container and keeping controlled fluid, typically water, which them from reaching the vacuum pump removes heat from a process Benefits Benefits Protects vacuum pumps Keeps water in the condenser at a For oil-sealed pumps, collection of vapors is critical to prevent them from getting into the vacuum pump where they would condense and contaminate the pump’s oil which will eventually cause stable low temperature thereby creat- loss of efficiency or irreparably damage pump ing ideal conditions for collecting the maximum amount of solvent Protects the environment For dry pumps, collection of vapors makes the evaporation system a closed system, preventing vapors from passing through the vacuum pump and into the environment Increases evaporation rate Vapors are collected as a frozen solid and are therefore not condensed inside the vacuum tub- ing, which would slow evaporation Combination Example In combination with a rotary evaporator In combination with a vacuum oven Exhaust Exhaust CF301+RE601+CA301+Vacuum pump DP63C+CA801+Vacuum pump Specifications Max. lowest Dehumidifying Capacity Recommended in Type Series temperature capacity (L) Features Application combination with Closed circulation system Used for many cooling Rotary evaporator Water CF301 N/A 4L Environment friendly coolant -

The Influence of Steam Cold Trap in the Vacuum Chamber Installation on the Ice Sublimation Speed1
ISNN 2083-1587; e-ISNN 2449-5999 2016,Vol. 20,No.2 , pp.5 3 -61 Agricultural Engineering DOI: 10.1515/agriceng-2016-0026 www.wir.ptir.org THE INFLUENCE OF STEAM COLD TRAP IN THE VACUUM CHAMBER INSTALLATION ON THE ICE SUBLIMATION SPEED1 Jarosław Diakun, Kamil Dolik, Adam Kopeć* Department of Processes and Devices of the Food Industry, Koszalin University of Technology ∗ Corresponding author: e-mail: [email protected] ARTICLE INFO ABSTRACT Article history: This work is a continuation of research on the differences in the Received: May 2015 sublimation speed of free ice and ice contained in the porous material. Received in the revised form: The results of previous research were published in Technica Agraria July 2015 12(1-2)/2013 (Diakun, Dolik, Kopec "The sublimation speed of free Accepted: September 2015 ice and ice in the sprat carcass"). A test stand used in studies was Key words: supplemented by a cold trap to prevent the steam flow into the vacu- steam cold trap, um pump and for the intensification of the ice sublimation process. sublimation, The comparative tests: with the cold trap and without were performed. ice, The research material (samples) was in the form of ice nugget, frozen sprat freeze-drying sprat carcasses and ice frozen within the sponge (porous material model). The aim of the study was to examine the cold trap impact on the conditions within the vacuum chamber during sublimation and the speed of the process. The differences in the sublimation speed for the free ice, the ice from the frozen sprat and from the model were rated.