Ammonium Sulfide Solution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
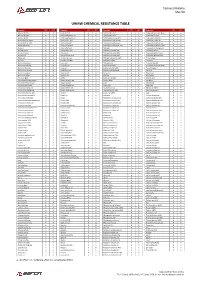
Chemical Properties
Technical Bulletin Mar/20 UHMW CHEMICAL RESISTANCE TABLE Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Acetic acid 10% A A Cetyl alcohol A A Hydrobromic acid A A Potassium ferrocyanide sat. A A Acetic acid 100% A B Chlorinated water 2% A A Hydrobromic acid B X Potassium fluoride A A Acetic acid 60% A A Chlorinated water sat. A B Hydrobromic acid aq.50% A A Potassium hydroxide A A Acetic aldehyde 100% B X Chlorine (dry gas) B X Hydrochloric acid aq.10% A A Potassium nitrate sat. A A Acetic aldehyde 40% B X Chlorine (liquid) B X Hydrocyanic acid aq.sat. A A Potassium perborate sat. A A Acetic anhydride A B Chlorine (wet gas) B X Hydrofluoric acid aq.40-75% A A Potassium perchlorate 10% A A Acetone A A ChlorineBenzene B X Hydrogen A A Potassium permanganate A A Acetophenone B A Chloroacetic acid X X Hydrogen bromide 10% A A Potassium sulfite A A Acrylic emulsion A A Chloroform X X Hydrogen peroxide 30% A A Potassium sulphate conc. A A Acrylonitrile A A Chlorosulfonic acid X X Hydrogen peroxide 90% A B Potassium sulphide conc. A A Adipic acid A A Chrome alum sat. A A Hydrogen phosphite 100% A A Propane (gas) A A Alumens A A Chromic acid 80% A A Hydrogen sulfide A A Propanol A A Aluminum acetate A A Citric acid A A Hydroquinone A A Propargyl alcohol A A Aluminum chloride A A Citronella oil B X Iodine (in alcohol) B B Propylene dichloride 100% X X Aluminum fluoride A A Clove oil A B Isobutyl alcohol 100% A A Propylene glycol A A Aluminum hydroxide A A Coclohexanona B X Isopropyl alcohol 100% A A Pyridine A B Aluminum oxalate A A Coconut oil A A Kerosene A B Resorcinol A A Aluminum sulfate A A Cod liver oil A A Ketchup A A Royal water B B Ammonia (gas) A A Coffee A A Lactic acid 10-90% A A Salicylic acid A A Ammoniacal ferrous citrate A A Copper chloride sat. -

APP202482 APP202482 Application Form Final.Pdf(PDF, 920
Application for the modified reassessment of a hazardous substance Under Section 63A of the Hazardous Substances and New Organisms Act 1996 Chemical Review 2012 – 2014 A modified reassessment of a range of substances for which new information was obtained in the period 2012 - 2014 Application number: APP202482 Applicant: Chief Executive, Environmental Protection Authority www.epa.govt.nz Chemical Review 2012 – 2014 (APP202482) 2 Applicant’s details Name: Rob Forlong, Chief Executive Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5403 Fax: 04 914 0433 Email: [email protected] Applicant’s contact person Name: Asela Atapattu Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5463 Fax: 04 914 0433 Email: [email protected] Signature of Applicant 3 June 2015 Rob Forlong Date Chief Executive Environmental Protection Authority Chemical Review 2012 – 2014 (APP202482) 3 Background The Environmental Protection Authority regularly receives new information from stakeholders regarding the classifications and controls of substances. EPA staff also note where changes to approvals are needed. Where those changes are not minor or technical, these changes require a reassessment or a modified reassessment of the approval of the substance under the HSNO Act 1996 (“the Act”) The Chemical Review is intended as a means of making changes to a number of approvals at once, taking into account the new information available to the EPA. This is undertaken as a modified reassessment under section 63A of the Act. This application makes recommendations to change some or all of the following aspects of the approvals in this application: - The approval name of the substance - The hazard classification(s) applied to the substance - The controls applied to the substance The controls changes proposed are largely as a result of changes to the hazard classifications of the substances in this application. -

Toxicological Profile for Hydrogen Sulfide and Carbonyl Sulfide
HYDROGEN SULFIDE AND CARBONYL SULFIDE 149 6. POTENTIAL FOR HUMAN EXPOSURE 6.1 OVERVIEW Hydrogen sulfide has been found in at least 34 of the 1,832 waste sites that have been proposed for inclusion on the EPA National Priorities List (NPL) and carbonyl sulfide was detected in at least 4 of the 1,832 waste sites (ATSDR 2015). However, the number of sites evaluated for these substances is not known and hydrogen sulfide and carbonyl sulfide are ubiquitous in the atmosphere. The frequency of these sites can be seen in Figures 6-1 and 6-2. Carbonyl sulfide and hydrogen sulfide are principal components in the natural sulfur cycle. Bacteria, fungi, and actinomycetes (a fungus-like bacteria) release hydrogen sulfide during the decomposition of 2- sulfur containing proteins and by the direct reduction of sulfate (SO4 ). Hydrogen sulfide is also emitted from volcanoes, stagnant or polluted waters, and manure or coal pits with low oxygen content (Aneja 1990; Khalil and Ramussen 1984). The majority of carbonyl sulfide that enters the environment is released to air and it is very abundant in the troposphere (Conrad and Meuser 2000; EPA 1994c, 1994d; Meinrat et al. 1992; Simmons et al. 2012; Stimler et al. 2010). It enters the atmosphere from both natural and anthropogenic sources (EPA 1994c, 1994d; Meinrat et al. 1992; Stimler et al. 2010). Carbonyl sulfide is released from wetlands, salt marshes, soil, oceans, deciduous and coniferous trees, and volcanic gases (Blake et al. 2004; EPA 1994c, 1994d; Meinrat et al. 1992; Rasmussen et al. 1982a, 1982b; Stimler et al. -
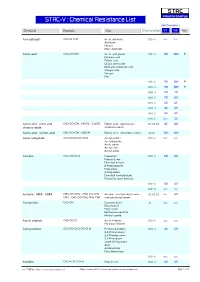
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Chemical Resistance Guide
CATALOG C-CRG-0517 VALVES Pressure-rated bronze, iron and alloy-iron gate, globe and check valves • Pressure- rated bronze ball valves • Boiler specialty valves • Commercial and industrial butterfly valves • Lined butterfly valves • Circuit balancing valves and kits • Carbon and stainless steel ball valves • ANSI flanged steel ball valves • Lined ball valves • Pneumatic and electric actuators and controls • Grooved ball and butterfly valves • High performance butterfly valves • UL/FM fire protection valves • MSS specification valves • Bronze specialty valves • Low pressure gate, globe, -GU check and ball valves • Frostproof sillcocks • Quarter-turn supply stops • Quarter- EM ID turn low pressure valves • PVC and CPVC plumbing and industrial ball valves • H Bronze and iron y-strainers • Sample valves • Sanitary valves • Lead-free valves E • Hydronic valves • Labor saving valves • Manifold systems • Water temperature C control valves • System quality valves • Press x PEX transition valves FITTINGS Wrot and cast copper pressure and drainage fittings • Cast copper alloy flanges • Powder coated steel companion flanges • Wrot and cast press fittings • ABS and PVC DWV fittings • Schedule 40 PVC pressure fittings • CPVC CTS fittings • CPVC CTS-to-metal transition fittings • Schedule 80 PVC and CPVC systems • Lead-free fittings • Press x PEX transition fittings • Cast bronze push fittings LEAD-FREE: Weighted average lead content ≤0.25% FLEXIBLE PIPING SYSTEMS PE-RT and PEX tubing for potable and radiant applications • Insulated tubing • Risers -

SAFETY DATA SHEET Sodium Hydrosulfide Solution
SAFETY DATA SHEET Sodium Hydrosulfide Solution Section 1. Identification Product name : Sodium Hydrosulfide Solution Synonyms : NaHS, sodium hydrogen sulfide, sodium bisulfide, sodium mercaptan Relevant identified uses of the substance or mixture and uses advised against Product use : Industrial Manufacturer : HollyFrontier Refining & Marketing LLC 2828 North Harwood Suite 1300 Dallas, Texas 75201 USA Customer Service: (888) 286-8836 Emergency telephone : CHEMTREC® (800) 424-9300 number CCN 201319 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : CORROSIVE TO METALS - Category 1 substance or mixture ACUTE TOXICITY (oral) - Category 4 SKIN CORROSION - Category 1 SERIOUS EYE DAMAGE - Category 1 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May be corrosive to metals. Harmful if swallowed. Causes severe skin burns and eye damage. Precautionary statements Prevention : Wear protective gloves. Wear eye or face protection. Wear protective clothing. Keep only in original container. Do not eat, drink or smoke when using this product. Wash hands thoroughly after handling. Response : Absorb spillage to prevent material damage. IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. Immediately call a POISON CENTER or physician. IF SWALLOWED: Immediately call a POISON CENTER or physician. Rinse mouth. Do NOT induce vomiting. IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water or shower. Wash contaminated clothing before reuse. Immediately call a POISON CENTER or physician. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. -

Technical Guide for Solutions of Sodium Hydrosulfide Technical Guide for Solutions of Sodium Hydrosulfide
TECHNICAL GUIDE FOR SOLUTIONS OF SODIUM HYDROSULFIDE TECHNICAL GUIDE FOR SOLUTIONS OF SODIUM HYDROSULFIDE TABLE OF CONTENTS TOPIC PAGE Overview 1 Health Hazards and First Aid 2 Flammability and Fire Response / Storage 3 Handling (PPE) 4 Equipment Recommendations / Transfers 5 Shipping 13 Releases 14 APPENDIX Material Safety Data Sheet 16 H2S Monitors 23 Hydrogen Sulfide Toxicity Chart 26 Density, Boiling and Freezing Points of 28 Sodium Hydrosulfide Viscosity of Typical 45% Sodium 30 Hydrosulfide Solution Sodium Hydrosulfide Site Assessment 32 Checklist TECHNICAL GUIDE FOR SOLUTIONS OF SODIUM HYDROSULFIDE Overview Sodium Hydrosulfide, chemical formula NaHS, is a highly alkaline salt solution with a pH of 11.5 to 12.5. The solution is typically yellow to dark green and has a rotten-egg odor due to the Hydrogen Sulfide (H2S) content. The product strength ranges from 20% to 45% by weight and weighs 9 to 11 pounds per gallon (specific gravity from 1.13 to 1.30 g/cm3). Solutions of NaHS are considered stable in normal transportation. The vapor space over NaHS solutions contains highly toxic Hydrogen Sulfide gas. The Hydrogen Sulfide gas is colorless and it is heavier than air. It will remain close to the ground and collect in low lying areas. The amount of Hydrogen Sulfide gas evolved from NaHS solutions is noticeably increased when the pH of the solution is below the pH of 10.2. This hap pens when the solution comes into contact with acidic materials or other materials that have a pH lower than 10.2. Dilution of the material will also create a minimal amount of Hydrogen Sulfide gas due to the lower pH of water coming in contact with the solution. -

Fate of Sodium Sulfide.Pdf
---· fate of SodiuM Sulfide and SodiuM ·sisul'ticte . ' 3 in SteaM Muffler Effluent ·,·:,· ~Jr l'f·;'.lfR & -..... _, __ . --.-- _,_,-; .'~;ENT The cheMical species generated by the treatMent of geotherMal steaM with sodiuM hydroxide <HaOH> include sodiuM carbonate <Haf0 3>, sodiuM bicarbonate <HaHCO?, sodiuM sulfide <HazS>, and sodiuM bisulfide <HaHS>. The forMer t~o cheMicals are environMentally benign and their disposal will have no significant effect on groundwater quality. SodiuM sulfide and bisulfide can 7 however~ have substantial iMpacts on water quality due to their toxicity at Moderate concentrations and their potential for odor generation at extreMely low concentrations. Hence 7 the fate of these cheMicals under norMal environMental conditions is of concern in considering their production and disposal as a result of treatMent of geotherMal steaM. Hydrogen sulfide abateMent froM geotherMal steaM has been accoMplished in Hawaii by the injection of a lOX solution of sodiuM hydroxide into the steaM phase at a Mole ratio of three Moles of sodiuM hydroxide to one Mole of hydrogen sulfide. The pH of the resultant solution will be in the range of 12 to 13 and hence the predoMinant sulfide species in solution will be sodiuM sulfide- Because a quantitative reaction of hydrogen sulfide to sodiuM sulfide ~auld require only two Moles of caustic to one MOle of sulfide~ an excess of caustic will be present that will Maintain the pH at a high level for a substantial period after the scrubber effluent is exposed to atMospheric air. Upon exposure of the sodiuM sulfide solution to air 7 the Most iMportant reaction that will occur is the oxidation of the sodiuM sulfide. -

In Situ Continuous Measurement of Dissolved Sulfide in Sewer Systems
In Situ Continuous Measurement of Dissolved Sulfide in Sewer Systems L Sutherland-Stacey 1, 2, S. Corrie 3, A. Neethling 2, I. Johnson 3, O. Gutierrez 4, R. Dexter 2, Z. Yuan 4, J. Keller 4, G. Hamilton 3 1 Physics Department, University of Auckland, Symonds Street, Auckland (Email: [email protected] ) 2 DCM Process Control, (Email: [email protected] ; [email protected] ; [email protected] ) 3 Gold Coast City Council (Email: [email protected]; [email protected]; [email protected] ) 4 Advanced Wastewater Management Centre, University of Queensland, (Email: [email protected]; [email protected]; [email protected] ) Abstract Sulfides are particularly problematic in the sewage industry. Hydrogen sulfide causes corrosion of concrete infrastructure, is dangerous at high concentrations and foul smelling at low concentrations. Despite the importance of sulfide monitoring there is no commercially available system to quantify sulfide in situ in waste water. In this article we report on our use of an s::can spectro::lyser TM to quantify bisulfide in waste water and additional analysis with a pH probe to calculate total dissolved sulfide. Our results show it is possible to use existing commercially available and field proven sensors to measure sulfide to mg/L levels continuously with little operator intervention and no sample preparation. Keywords: In-situ, on-line, sulfide, septic sewage, real-time INTRODUCTION Hydrogen sulfide (H 2S) is generated in the aqueous phase of wastewater by bacterial reduction of sulfate under anaerobic conditions (Tanaka et al. , 2000). -

Sodium Hydrogen Sulfide
Product Safety Summary Sodium Hydrogen Sulfide, Solid (70-72% with Crystallization Waters < 25%) CAS No. 16721-80-5 This Product Safety Summary is intended to provide a general overview of the chemical substance. The information on the summary is basic information and is not intended to provide emergency response information, medical information or treatment information. The summary should not be used to provide in-depth safety and health information. In-depth safety and health information can be found on the Safety Data Sheet (SDS) for the chemical substance. Names Sodium hydrogen sulfide (sulphide) Sodium hydrosulfide (hydrosulphide) Sodium mercaptan Sodium sulfhydrate Sodium bisulfide Sodium mercaptide Product Overview Solvay Fluorides, LLC does not sell sodium hydrogen sulfide directly to consumers. Consumers are unlikely to be exposed to sodium hydrogen sulfide in any of the consumer product applications listed below and only where the sodium hydrogen sulfide is not transformed or reacted. Sodium hydrogen sulfide is a yellow, solid flake with a sulfurous (rotten egg) smell. It is used in water treatment, the pulp and paper industry, and in leather processing as a tanning agent or hair remover (from hides). Sodium hydrogen sulfide may be used in the making of colors and dyes. It can also be used in the manufacture of other chemicals, metals or in ore processing (mining) and in waste water, soil and process sludge treatment. Exposure to sodium hydrogen sulfide can cause severe irritation to the skin, eyes, and respiratory tract. Sodium hydrogen sulfide may cause sensitization (develop an allergic reaction). Breathing sodium hydrogen sulfide dusts may aggravate asthma or other pulmonary (breathing) diseases and may cause headaches, dizziness, nausea and vomiting. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Sulfoxide Directed Metal-Free Cross Coupling: Propargylation of Aromatic and Heteroaromatic Systems
Sulfoxide Directed Metal-free Cross Coupling: Propargylation of Aromatic and Heteroaromatic Systems A dissertation submitted to The University of Manchester for the degree of Master of Science by Research in the Faculty of Engineering and Physical Science. 2015 Yuntong Zhang School of Chemistry Contents List of Tables: ............................................................................... 4 List of Abbreviations ..................................................................... 5 Abstract ......................................................................................... 9 Declaration .................................................................................. 10 Copyright Statement .................................................................... 11 Acknowledgement ....................................................................... 12 Chapter 1: Introduction ............................................................... 13 1.1 Pummerer and Pummerer-type Reactions............................ 13 1.1.1 The Classical Pummerer Rearrangement .................... 13 1.1.2 Additive and Vinylogous Pummerer Reactions .......... 14 1.1.3 Interrupted Pummerer Reactions ................................ 18 1.2 Pummerer-Type Reactions Extended to Aromatic Systems . 24 1.2.1 Aromatic and Hetero-aromatic Pummerer-type Reactions ................................................................................. 24 1.2.2 Ortho Alkylations of Aryl and Heteroaryl Systems .... 29 1.3 Beyond Classical Pummerer Reaction Electrophiles