Hazardous Waste Management Guide
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

United States Patent Office Patented Sept
3,000,702 United States Patent Office Patented Sept. 19, 196 2 since potassium fluoride is very soluble. In this case, a 3,000,702 MANUEFACTURE OF SODIUM FLUOR DE double or complex salt is formed in the melt between George L. Cunningham, Burtonsville, Md., assignor to potassium fluoride and the calcium salt and that is slowly W. R. Grace & Co., New York, N.Y., a corporation extracted with water in spite of the great solubility of of Connecticut potassium fluoride in water. The corrosion of the molten No Drawing. Filed May 23, 1958, Ser. No. 737,196 melt is also a considerable problem although suitable 4. Claims. (CI. 23-88) ceramic materials have been discovered for constructing the fusion vessel. This invention relates to a process for the manufac Processes have been devised for the production of so ture of sodium fluoride relatively low in silica content. 10 dium fluoride based on the thermal decomposition of More particularly, this invention relates to a cyclic proc sodium silicofluoride to sodium fluoride and silicon tetra ess whereby sodium fluoride, relatively low in silica con fluoride (U.S. 1,896,697, U.S. 2,588,786, 1,730,915, tent and ammonium chloride, may be manufactured from 2,602,726, and U.S. 1,664,348). These processes are cheap and readily available raw materials. faulty in that relatively high temperatures are required to As manufactured at the present time, technical sodium 15 decompose the sodium silicofluoride and thus it is very fluoride contains 94% to 97% NaF and 1.5% to 5.0% difficult to carry the reaction to completion. -
Chemical Properties
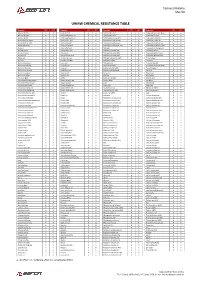
Technical Bulletin Mar/20 UHMW CHEMICAL RESISTANCE TABLE Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Reagente 23°C 60°C Acetic acid 10% A A Cetyl alcohol A A Hydrobromic acid A A Potassium ferrocyanide sat. A A Acetic acid 100% A B Chlorinated water 2% A A Hydrobromic acid B X Potassium fluoride A A Acetic acid 60% A A Chlorinated water sat. A B Hydrobromic acid aq.50% A A Potassium hydroxide A A Acetic aldehyde 100% B X Chlorine (dry gas) B X Hydrochloric acid aq.10% A A Potassium nitrate sat. A A Acetic aldehyde 40% B X Chlorine (liquid) B X Hydrocyanic acid aq.sat. A A Potassium perborate sat. A A Acetic anhydride A B Chlorine (wet gas) B X Hydrofluoric acid aq.40-75% A A Potassium perchlorate 10% A A Acetone A A ChlorineBenzene B X Hydrogen A A Potassium permanganate A A Acetophenone B A Chloroacetic acid X X Hydrogen bromide 10% A A Potassium sulfite A A Acrylic emulsion A A Chloroform X X Hydrogen peroxide 30% A A Potassium sulphate conc. A A Acrylonitrile A A Chlorosulfonic acid X X Hydrogen peroxide 90% A B Potassium sulphide conc. A A Adipic acid A A Chrome alum sat. A A Hydrogen phosphite 100% A A Propane (gas) A A Alumens A A Chromic acid 80% A A Hydrogen sulfide A A Propanol A A Aluminum acetate A A Citric acid A A Hydroquinone A A Propargyl alcohol A A Aluminum chloride A A Citronella oil B X Iodine (in alcohol) B B Propylene dichloride 100% X X Aluminum fluoride A A Clove oil A B Isobutyl alcohol 100% A A Propylene glycol A A Aluminum hydroxide A A Coclohexanona B X Isopropyl alcohol 100% A A Pyridine A B Aluminum oxalate A A Coconut oil A A Kerosene A B Resorcinol A A Aluminum sulfate A A Cod liver oil A A Ketchup A A Royal water B B Ammonia (gas) A A Coffee A A Lactic acid 10-90% A A Salicylic acid A A Ammoniacal ferrous citrate A A Copper chloride sat. -

Tutorial 2 FORMULAS, PERCENTAGE COMPOSITION
T-6 Tutorial 2 FORMULAS, PERCENTAGE COMPOSITION, AND THE MOLE FORMULAS: A chemical formula shows the elemental composition of a substance: the chemical symbols show what elements are present and the numerical subscripts show how many atoms of each element there are in a formula unit. Examples: NaCl: one sodium atom, one chlorine atom in a formula unit CaCl2: one calcium atom, two chlorine atoms in a formula unit Mg3N2: three magnesium atoms, two nitrogen atoms in a formula unit The presence of a metal in a chemical formula indicates an ionic compound, which is composed of positive ions (cations) and negative ions (anions). A formula with only nonmetals indicates a + molecular compound (unless it is an ammonium, NH4 , compound). Only ionic compounds are considered in this Tutorial. There are tables of common ions in your lecture text, p 56 (cations) and p 57 (anions). A combined table of these same ions can be found on the inside back cover of the lecture text. A similar list is on the next page; all formulas needed in this and subsequent Tutorial problems can be written with ions from this list. Writing formulas for ionic compounds is very straightforward: TOTAL POSITIVE CHARGES MUST BE THE SAME AS TOTAL NEGATIVE CHARGES. The formula must be neutral. The positive ion is written first in the formula and the name of the compound is the two ion names. EXAMPLE: Write the formula for potassium chloride. The name tells you there are potassium, K+, and chloride, Cl–, ions. Each potassium ion is +1 and each chloride ion is -1: one of each is needed, and the formula for potassium chloride is KCl. -

COMBINED LIST of Particularly Hazardous Substances
COMBINED LIST of Particularly Hazardous Substances revised 2/4/2021 IARC list 1 are Carcinogenic to humans list compiled by Hector Acuna, UCSB IARC list Group 2A Probably carcinogenic to humans IARC list Group 2B Possibly carcinogenic to humans If any of the chemicals listed below are used in your research then complete a Standard Operating Procedure (SOP) for the product as described in the Chemical Hygiene Plan. Prop 65 known to cause cancer or reproductive toxicity Material(s) not on the list does not preclude one from completing an SOP. Other extremely toxic chemicals KNOWN Carcinogens from National Toxicology Program (NTP) or other high hazards will require the development of an SOP. Red= added in 2020 or status change Reasonably Anticipated NTP EPA Haz list COMBINED LIST of Particularly Hazardous Substances CAS Source from where the material is listed. 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide Acutely Toxic Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- Acutely Toxic 1-(2-Chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (Methyl-CCNU) Prop 65 KNOWN Carcinogens NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) IARC list Group 2A Reasonably Anticipated NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) (Lomustine) Prop 65 1-(o-Chlorophenyl)thiourea Acutely Toxic 1,1,1,2-Tetrachloroethane IARC list Group 2B 1,1,2,2-Tetrachloroethane Prop 65 IARC list Group 2B 1,1-Dichloro-2,2-bis(p -chloropheny)ethylene (DDE) Prop 65 1,1-Dichloroethane -

Comments of Teresa Homan with Attachments
Teresa Homan Watertown, SD 57201 I am a landowner in Deuel County, South Dakota. Our land boarders the Deuel Harvest Wind Project in Deuel county, Docket # EL 18-053. There are 112 towers cited in the project, with 9 towers within a mile of our property. We have spent over three decades developing this property to enhance wildlife and for the enjoyment of our family. Can you imagine how we felt when we found we have a population of eastern bluebirds? We have yellow warblers, which by the way feed on the web worms that form in our trees. We have orioles, cedar waxwings, brown thrashers, rose breasted grosbeaks, gold finches, purple finches, robins, blue jays, nuthatches, eastern kingbirds, bitterns, dark eyed juncos, red winged blackbirds, morning doves, owls, cow birds, northern mocking birds, grey cat birds, wood thrushes, tufted titmouse, king fishers, indigo buntings, scarlet tanagers, bobolinks, meadowlarks, many woodpeckers, turkeys, turkey vultures, even humming birds and bald eagles. There are more, just to numerous to list. Many of these birds we have seen for the first time in our lives on this property in the past 1 O years. Not only are these birds beautiful and fun to watch, they have their purpose in the ecosystem. We also see northern long eared bats, that are on the endangered list in South Dakota. These birds are making a come back after the use of insecticides that nearly wiped out many. In the 1940's the insecticide DDT was introduced for public use, it is now banned from sale. In 1976 the herbicide Roundup was introduced to the public. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0027386 A1 Kurihara Et Al
US 20110027386A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0027386 A1 Kurihara et al. (43) Pub. Date: Feb. 3, 2011 (54) ANTMICROBAL. ZEOLITE AND (30) Foreign Application Priority Data ANTMICROBAL COMPOSITION Feb. 22, 2006 (JP) ................................. 2006-045241 (75) Inventors: Yasuo Kurihara, Nagoya-shi (JP); Kumiko Miyake, Nagoya-shi (JP); Publication Classification Masashi Uchida, Nagoya-shi (JP) (51) Int. Cl. Correspondence Address: AOIN 59/6 (2006.01) NIXON & VANDERHYE, PC COB 39/02 (2006.01) 901 NORTH GLEBE ROAD, 11TH FLOOR AOIP I/00 (2006.01) ARLINGTON, VA 22203 (US) (52) U.S. Cl. .......................... 424/618; 423/701; 423/700 (73) Assignee: Sinanen Zeomic Co., Ltd., (57) ABSTRACT Nagoya-Shi (JP) The present invention relates to antimicrobial zeolite which comprises zeolite whereina hardly soluble zinc salt is formed (21) Appl. No.: 12/923,854 within fine pores present therein and an antimicrobial com position which comprises the foregoing antimicrobial Zeolite (22) Filed: Oct. 12, 2010 in an amount ranging from 0.05 to 80% by mass. The antimi crobial Zeolite according to the present invention can widely Related U.S. Application Data be applied, without causing any color change, even to the (63) Continuation of application No. 1 1/705,460, filed on goods which undergo color changes with the elapse of time Feb. 13, 2007. when the conventional antimicrobial zeolite is added. US 2011/002738.6 A1 Feb. 3, 2011 ANTMICROBAL. ZEOLITE AND 3. An antimicrobial composition comprising the foregoing ANTMICROBAL COMPOSITION antimicrobial zeolite as set forth in the foregoing item 1 or 2 in an amount ranging from 0.05 to 80% by mass. -

APP202482 APP202482 Application Form Final.Pdf(PDF, 920
Application for the modified reassessment of a hazardous substance Under Section 63A of the Hazardous Substances and New Organisms Act 1996 Chemical Review 2012 – 2014 A modified reassessment of a range of substances for which new information was obtained in the period 2012 - 2014 Application number: APP202482 Applicant: Chief Executive, Environmental Protection Authority www.epa.govt.nz Chemical Review 2012 – 2014 (APP202482) 2 Applicant’s details Name: Rob Forlong, Chief Executive Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5403 Fax: 04 914 0433 Email: [email protected] Applicant’s contact person Name: Asela Atapattu Address: EPA, Level 10, 215 Lambton Quay, Private Bag 63002, Wellington 6140 Phone: 04 474 5463 Fax: 04 914 0433 Email: [email protected] Signature of Applicant 3 June 2015 Rob Forlong Date Chief Executive Environmental Protection Authority Chemical Review 2012 – 2014 (APP202482) 3 Background The Environmental Protection Authority regularly receives new information from stakeholders regarding the classifications and controls of substances. EPA staff also note where changes to approvals are needed. Where those changes are not minor or technical, these changes require a reassessment or a modified reassessment of the approval of the substance under the HSNO Act 1996 (“the Act”) The Chemical Review is intended as a means of making changes to a number of approvals at once, taking into account the new information available to the EPA. This is undertaken as a modified reassessment under section 63A of the Act. This application makes recommendations to change some or all of the following aspects of the approvals in this application: - The approval name of the substance - The hazard classification(s) applied to the substance - The controls applied to the substance The controls changes proposed are largely as a result of changes to the hazard classifications of the substances in this application. -

Equimax & Eraquell Oral Gel for Horses
Equimax & Eraquell Oral Gel for Horses Annual Wormer Pack [active ingredients: Ivermectin & Praziquantel] (POM-VPS) Revised AN Equimax Oral Gel for Horses January 2013 01009/2012 Eraquell Oral Gel for Horses December 2015 01163/2015 Page 1 of 15 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Equimax Oral Gel for Horses 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of Equimax contains Active substances Ivermectin ........................................................ 18.7 mg Praziquantel ..................................................... 140.3 mg Excipients Titanium dioxide (E171) ................................... 20 mg Propylene glycol ............................................... 731 mg For a full list of excipents, see section 6.1 3. PHARMACEUTICAL FORM Oral gel. 4. CLINICAL PARTICULARS 4.1 Target species Horses. 4.2 Indications for use, specifying the target species For the treatment of mixed cestode and nematode or arthropod infestations, due to adult and immature roundworms, lungworms, bots and tapeworms in horses: Nematodes Large-strongyle: Strongylus vulgaris (adult and arterial larvae) Strongylus edentatus (adult and L4 tissue larval stages) Strongylus equinus (adult) Triodontophorus spp. (adult) Small-strongyle: Cyathostomum: Cylicocyclus spp., Cylicostephanus spp., Cylicodontophorus spp., Gyalocephalus spp. (adult and non-inhibited mucosal larvae). Parascaris: Parascaris equorum (adult and larvae). Page 2 of 15 Oxyuris: Oxyuris equi (larvae). Trichostrongylus:Trichostrongylus -

Comparison of Acute Noaels and Benchmark Doses for Female Brain Cholinesterase Inhibition
Supplemental Material for: February 5-8, 2002 SAP 25 January 2002 Comparison of Acute NOAELs and Benchmark Doses for Female Brain Cholinesterase Inhibition In cumulative risk assessment, it is important to characterize both the time frame for exposure (e.g., What is the exposure duration?) and for the toxic effect (e.g., What are the time to peak effects and the time to recovery?). In the Preliminary Cumulative Risk Assessment of the Organophoshate Pesticides (OPs) relative potency factors (RPFs) for 29 chemicals and points of departure (PODs) and the index chemical were determined based on whole brain cholinesterase (ChE) data from toxicity studies of 21 days and longer. The Office of Pesticide Programs has argued that the use of steady state data for relative potency determination generates relative potency factors (RPFs) that are reproducible and reflect less variability than RPFs derived from single-dose or short-term studies where the extent of inhibition changes rapidly immediately following dosing. OPP has posed a question to the FIFRA SAP for the February 5-8, 2002 review concerning how best to evaluate risk, taking into account the temporal characteristics of the hazard endpoint (i.e., cholinesterase inhibition) and the temporal characteristics of the exposure patterns for the food, drinking water, and residential/nonoccupational pathways. In order to facilitate the panel discussion, a table listing the available single dose toxicity studies performed with OPs has been made. Most of the studies are acute neurotoxicity (ACN) studies (OPPT Guideline 870.6200, OPP Guideline 81-8) administered by gavage. Acute lethality studies were not included. Dose levels, no- observed-adverse-effect levels (NOAELs), and no-observed-adverse-effect levels (LOAELs) for female brain ChE are also listed in the table. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

A Study on Physical Chemistry of Solid a Mmonium Materials for Nox Reduction of Diesel Engine Emissions
A Study on Physical Chemistry of Solid A mmonium Materials for NOx Reduction of Diesel Engine Emissions Cheon Seog (Steve) Yoon and Jong Kook Shin Hannam University, Daejeon, KOREA Hoyeol Lee and Hongsuk Kim Korea Institute of Machinery & Materials, Daejeon, KOREA 2014 DOE CLEERS Workshop University of Michigan, Dearborn, MI, USA 1 Table of Contents • Introduction of Solid SCR System • Ammonium Salts • Chemical Reactions, Decomposition Chemistry • Chemical Kinetic Parameters by TGA, DTA and DSC • Decomposition Rate from Hot Plate Test and Chemical Kinetic Parameters • Simple Reactor with Visible Window • Equilibrium Vapor Pressure Curve for Ammonium Carbonate • Acquisition of Re-solidified Materials from Ammonium Carbonate • Analytical Study of Re-solidified Materials from Ammonium Carbonate by XRD, FT-IR, and EA • Concluding Remarks • Acknowledgement • Reference 2 Solid SCR System • NOx purification technology by using NH3, which is generated from solid ammonium. • Ammonium carbonate, (NH4)2CO3 , is solid at room temperature, and it decomposes into NH3, H2O & CO2 above temperature of 60℃. 3 Material Properties of Ammonium Salts Solid urea Ammonium carbonate Ammonium cabarmate Molecular formula (NH2)2CO (NH4)2CO3 NH2COONH4 Molecular weight 60.07 96.09 78.07 3 Density, g/cm 1.33 1.5 1.6 Mols NH3 per Mol 2 2 2 Mols NH3 per kg 33.3 20.8 25.6 Decomposition temp., ℃ 140 58 60 NH2CONH2↔ NH3+HNCO Reaction mechanism (NH4)2CO3↔2NH3+CO2+H2O NH4COONH2 ↔ 2NH3 + CO2 HNCO +H2O ↔ NH3 + CO2 Cost cheap cheap moderate * HNCO: Isocyanic Acid [ref] G. Fulks, -

Chemical Resistance: Deco-Trowel
CHEMICAL RESISTANCE DECO-TROWEL ® SERIES 223 Tnemec Company, Inc. 6800 Corporate Drive Kansas City, Missouri 64120-1372 +1 816-483-3400 www.tnemec.com © December 16, 2019 by Tnemec Company, Inc. Chem223 Page 1 of 19 CHEMICAL RESISTANCE DECO-TROWEL ® | SERIES 223 COMMON PROBLEM AREAS FOR COATINGS AND SOLUTIONS Problem: Coating Solution: Points of failure Carefully and due to thin spots fully coat in coating Problem: Rough Pinhole Solution: Uneven Undercut Grind smooth welds Problem: Gaps between Solution: plates, coating Continuous can not cover welds Problem: Gaps between Solution: plates, coating Continuous can not cover welds Problem: Coating Sharp surface Solution: contours create Round the thin spots in contours coating Problem: Skip welding Solution: creates gaps Continuous that coating welds can not cover Problem: Skip welding Solution: creates gaps Continuous that coating welds can not cover 2 channels back to back IMPORTANT: Definitions for the terms and acronyms used in this guide to describe the recommended exposures, along with other important information, can be found on the cover page of this guide or by contacting Tnemec Technical Service. Coatings should not be applied in a chemical exposure environment until the user has thoroughly read and understood the product information and full project details have been discussed with Tnemec Technical Service. Tnemec Company, Inc. 6800 Corporate Drive Kansas City, Missouri 64120-1372 +1 816-483-3400 www.tnemec.com © December 16, 2019 by Tnemec Company, Inc. Chem223 Page 2 of 19 CHEMICAL RESISTANCE DECO-TROWEL ® | SERIES 223 ¹ Product is NOT suitable for direct or indirect food contact. Intended Use and temperature information relates to product’s performance capabilities only.