Review Article Intracranial Pressure Monitoring: Invasive Versus Non-Invasive Methods—A Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

INDEX Abducent Neurons Anatomy 135 Clinical Signs 137 Diseases
INDEX Abducent neurons Anatomy 135 Clinical signs 137 Diseases 139 Function 135 Abiotrophic sensorineural deafness 438 Abiotrophy 100, 363 Auditory 438 Cerebellar cortical 363 Motor neuron 100 Nucleus ambiguus 159 Peripheral vestibular 336 Abscess-Brainstem 330 Caudal cranial fossa 343 Cerebellar 344 Cerebral 416, 418 Pituitary 162 Streptococcus equi 418 Abyssian cat-Glucocerebrosidosis 427 Myastheina gravis 93 Accessory neurons Anatomy 152 Clinical signs 153 Diseases 153 Acetozolamide 212 Acetylcholine 78, 169, 354, 468, 469 Receptor 78 Acetylcholinesterase 79 Achiasmatic Belgian sheepdog 345 Acoustic stria 434 Acral mutilation 237 Adenohypophysis 483 Releasing factors 483 Adenosylmethionine 262 Adhesion-Interthalamic 33, 476 Adiposogenital syndrome 484 Adipsia 458, 484 Adrenocorticotrophic hormone 485 Adversive syndrome 72, 205, 460 Afghan hound-Inherited myelinolytic encephalomyelopathy 264 Agenized flour 452 Aino virus 44 Akabane virus 43 Akita-Congenital peripiheral vestibular disease 336 Alaskan husky encephalopathy 522 Albinism, ocular 345 Albinotic sensorineural deafness 438 Alexander disease 335 Allodynia 190 Alpha fucosidosis 427 Alpha glucosidosis 427 Alpha iduronidase 427 Alpha mannosidase 427 Alpha melanotropism 484 Alsatian-idiopathic epilepsy 458 Alternative anticonvulsant drugs 466 American Bulldog-Ceroid lipofuscinosis 385, 428 American Miniature Horse-Narcolepsy 470 American StaffordshireTerrier-Cerebellar cortical abiotrophy 367 Amikacin 329 Aminocaproic acid 262 Aminoglycoside antibiotics 329, 439 Amprolium toxicity -
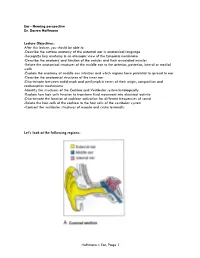
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

University of Groningen Dynamics of Inner Ear Pressure Change With
University of Groningen Dynamics of inner ear pressure change with emphasis on the cochlear aqueduct Laurens-Thalen, Elisabeth Othilde IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2004 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Laurens-Thalen, E. O. (2004). Dynamics of inner ear pressure change with emphasis on the cochlear aqueduct [S.l.]: [S.n.] Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 10-02-2018 DYNAMICS OF INNER EAR PRESSURE CHANGE WITH EMPHASIS ON THE COCHLEAR AQUEDUCT E.O. LAURENS - THALEN Stellingen behorende bij het proefschrift Dynamics of Inner Ear Pressure Change with Emphasis on the Cochlear Aqueduct E.O. Laurens - Thalen Groningen, 14 april 2004 1. De stromingsweerstand van de aquaductus cochlearis is variabel. -

High-Yield Embryology 4
LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page i Aptara Inc High-Yield TM Embryology FOURTH EDITION LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page ii Aptara Inc LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page iii Aptara Inc High-Yield TM Embryology FOURTH EDITION Ronald W. Dudek, PhD Professor Brody School of Medicine East Carolina University Department of Anatomy and Cell Biology Greenville, North Carolina LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page iv Aptara Inc Acquisitions Editor: Crystal Taylor Product Manager: Sirkka E. Howes Marketing Manager: Jennifer Kuklinski Vendor Manager: Bridgett Dougherty Manufacturing Manager: Margie Orzech Design Coordinator: Terry Mallon Compositor: Aptara, Inc. Copyright © 2010, 2007, 2001, 1996 Lippincott Williams & Wilkins, a Wolters Kluwer business. 351 West Camden Street 530 Walnut Street Baltimore, MD 21201 Philadelphia, PA 19106 Printed in China All rights reserved. This book is protected by copyright. No part of this book may be reproduced or transmitted in any form or by any means, including as photocopies or scanned-in or other electronic copies, or utilized by any information storage and retrieval system without written permission from the copyright owner, except for brief quotations embodied in critical articles and reviews. Materials appear- ing in this book prepared by individuals as part of their official duties as U.S. government employees are not covered by the above-mentioned copyright. To request permission, please contact Lippincott Williams & Wilkins at 530 Walnut Street, Philadelphia, PA 19106, via email at [email protected], or via website at lww.com (products and services). -

The Anatomy of the Head of Ctenosaura Pectinata (Iguanidae)
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 94 The Anatomy of the Head of Ctenosaura pectinata (Iguanidae) BY THOMAS M. OELRICH ANN ARBOR MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN March 21, 1956 LIST OF THE MISCELLANEOUS PUBLICATIONS OF THE MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN Address inquiries to the Director of the Museum of Zoology, Ann Arbor, Michigan *On sale from the University Press, 311 Maynard St., Ann Arbor, Michigan. Bound in Paper No. 1. Directions for Collecting and Preserving Specimens of Dragonflies for Museum Purposes. By E. B. Williamson. (1916) Pp. 15, 3 figures . No. 2. An Annotated List of the Odonata of Indiana. By E. B. Williamson. (1917) Pp. 12, 1 map . No. 3. A Collecting Trip to Colombia, South America. By E. B. Williamson. (1918) Pp. 24 (Out of print) No. 4. Contributions to the Botany of Michigan. By C. K. Dodge. (1918) Pp. 14 No. 5. Contributions to the Botany of Michigan, 11. By C. K. Dodge. (1918) Pp. 44, 1 map No. 6. A Synopsis of the Classification of the Fresh-water Mollusca of North America, North of Mexico, and a Catalogue of the More Recently Described Species, with Notes. By Bryant Walker. (1918) Pp. 213, 1 plate, 233 figures No. 7. The Anculosae of the Alabama River Drainage. By Calvin Goodrich. (1922) Pp. 57, 3 plates . No. 8. The Amphibians and Reptiles of the Sierra Nevada de Santa Marta, Colombia. By Alexander G. Ruthven. (1922) Pp. 69, 13 plates, 2 figures, 1 map No. 9. Notes on American Species of Triacanthagyna and Gynacantha. -

Thieme: Veterinary Anatomy of Domestic Animals
Index Page numbers in bold indicate references in figures. A Abducent nerve (VI) 521, 524, 528, Acetabulum 122, 243, 246, 247, Alar 550–551, 554, 560, 611, 615 247–250, 749 – canal 79 Abaxial 26 Abductor muscle/s 47 Acetylcholinase 65 – cartilages 398,402 – – 202 collateral ligament 200 201, , – of thigh 288, 292 Acetylcholine 44, 64–65, 581 –– ossification 730 204 Ablatio retinae 697 Achilles tendon 296–297, 302 – fold 402 – digital Abnormal behaviour 699 Acinous glands 67 – foramen 110, 115, 129–130, 626 –– 667 artery 665, Abomasal velum 361 Acoustic – ligament 130 –– 668 nerves Abomasopexy 715 – area 536 , 556 – notch 110 –– 667 veins Abomasum 360, 362, 366, 367, 378, – organs 524 Albino animals 697 – dorsal proper artery of fourth 720–721 – radiation 536 Albugineous tunic 660 digit 657, – blood supply 369 Acquired reflexes 63 – of penis 443, 446 – palmar – displacement 367, 715 Acrocoracohumeral ligament 750 – of testis 433 –– digital veins 665 – perigastric ring of arteries 370 Acromion 171, 173–174, 190 Albumen (egg white) 766 –– ligament of proximal interphalan- – projection onto lateral body wall 743 – fracture 727 Alpha cells 596 202 geal joint Absolute dullness 746 Acropodium 181, 244,259 Alular –– proper artery 727 Absorption of substances 67 ACTH (adrenocorticotropic – digit 748 –– proper artery of fourth digit Accessory hormone) 36, 588 – remiges 778, 778 660 657, – axillary lymph node 505, 507 Actin filaments 44 Alveolar –– proper nerve 727 – carpal bone 173, 182, 182–183,184, Adamantine 339 – border of mandible 90, 92 –– -

Central Nervous System. Sense Organs Study Guide
CENTRAL NERVOUS SYSTEM. SENSE ORGANS STUDY GUIDE 0 Ministry of Education and Science of Ukraine Sumy State University Medical Institute CENTRAL NERVOUS SYSTEM. SENSE ORGANS STUDY GUIDE Recommended by the Academic Council of Sumy State University Sumy Sumy State University 2017 1 УДК 6.11.8 (072) C40 Authors: V. I. Bumeister, Doctor of Biological Sciences, Professor; O. S. Yarmolenko, Candidate of Medical Sciences, Assistant; O. O. Prykhodko, Candidate of Medical Sciences, Assistant Professor; L. G. Sulim, Senior Lecturer Reviewers: O. O. Sherstyuk – Doctor of Medical Sciences, Professor of Ukrainian Medical Stomatological Academy (Poltava); V. Yu. Harbuzova – Doctor of Biological Sciences, Professor of Sumy State University (Sumy) Recommended by for publication Academic Council of Sumy State University as a study guide (minutes № 11 of 15.06.2017) Central nervous system. Sense organs : study guide / C40 V. I. Bumeister, O. S. Yarmolenko, O. O. Prykhodko, L. G. Sulim. – Sumy : Sumy State University, 2017. – 173 p. ISBN 978-966-657- 694-4 This study gnide is intended for the students of medical higher educational institutions of IV accreditation level, who study human anatomy in the English language. Навчальний посібник рекомендований для студентів вищих медичних навчальних закладів IV рівня акредитації, які вивчають анатомію людини англійською мовою. УДК 6.11.8 (072) © Bumeister V. I., Yarmolenko O. S., Prykhodko O. O, Sulim L. G., 2017 ISBN 978-966-657- 694-4 © Sumy State University, 2017 2 INTRODUCTION Human anatomy is a scientific study of human body structure taking into consideration all its functions and mechanisms of its development. Studying the structure of separate organs and systems in close connection with their functions, anatomy considers a person's organism as a unit which develops basing on the regularities under the influence of internal and external factors during the whole process of evolution. -

Imaging of Cerebrospinal Fluid Flow: Fundamentals, Techniques, and Clinical Applications of Phase-Contrast Magnetic Resonance Imaging
© Pol J Radiol 2019; 84: e240-e250 DOI: https://doi.org/10.5114/pjr.2019.86881 Received: 08.03.2019 Accepted: 15.03.2019 Published: 13.05.2019 http://www.polradiol.com Review paper Imaging of cerebrospinal fluid flow: fundamentals, techniques, and clinical applications of phase-contrast magnetic resonance imaging Adrian KorbeckiA,B,E, Anna ZimnyA,B,E, Przemysław PodgórskiA,B,D, Marek SąsiadekA,D,E, Joanna BladowskaA,B,E Department of General and Interventional Radiology and Neuroradiology, Wroclaw Medical University, Wroclaw, Poland Abstract Cerebrospinal fluid (CSF) is a dynamic compartment of the brain, constantly circulating through the ventricles and subarachnoid space. In recent years knowledge about CSF has expended due to numerous applications of phase- contrast magnetic resonance imaging (PC-MRI) in CSF flow evaluation, leading to the revision of former theories and new concepts about pathophysiology of CSF disorders, which are caused either by alterations in CSF production, absorption, or its hydrodynamics. Although alternative non-invasive techniques have emerged in recent years, PC-MRI is still a fundamental sequence that provides both qualitative and quantitative CSF assessment. PC-MRI is widely used to evaluate CSF hydrodyna- mics in normal pressure hydrocephalus (NPH), Chiari type I malformations (CMI), syringomyelia, and after neuro- surgical procedures. In NPH precisely performed PC-MRI provides reliable clinical information useful for differential diagnosis and selection of patients benefiting from surgical operation. Patients with CMI show abnormalities in CSF dynamics within the subarachnoid space, which are pronounced even further if syringomyelia coexists. Another indication for PC-MRI may be assessment of post-surgical CSF flow normalisation. -

Incidence of Unilateral, High Frequency, Sensorineural Hearing Loss in Shunt Treated Hydrocephalic Children Ipsilateral to Shunt Placement
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 12-4-2000 Incidence of Unilateral, High Frequency, Sensorineural Hearing Loss in Shunt Treated Hydrocephalic Children Ipsilateral to Shunt Placement Susan E. Spirakis University of South Florida Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the American Studies Commons Scholar Commons Citation Spirakis, Susan E., "Incidence of Unilateral, High Frequency, Sensorineural Hearing Loss in Shunt Treated Hydrocephalic Children Ipsilateral to Shunt Placement" (2000). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/1548 This Dissertation is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. University of South Florida Scholar Commons @USF Theses and Dissertations 6-1-2000 Incidence of unilateral, high frequency, sensorineural hearing loss in shunt treated hydrocephalic children ipsilateral to shunt placement Susan E. Spirakis University of South Florida Scholar Commons Citation Spirakis, Susan E., "Incidence of unilateral, high frequency, sensorineural hearing loss in shunt treated hydrocephalic children ipsilateral to shunt placement" (2000). Theses and Dissertations. Paper 1548. http://scholarcommons.usf.edu/etd/1548 This Dissertation is brought to you for free and open access by Scholar Commons @USF. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholar Commons @USF. For more information, please contact [email protected]. Incidence of Unilateral, High Frequency, Sensorineural Hearing Loss in Shunt Treated Hydrocephalic Children Ipsilateral to Shunt Placement Susan E. -
The Bony and the Membranous Labyrinth
The bony and the membranous labyrinth Zsuzsanna Tóth, PhD Semmelweis University, Department of Anatomy, Histology and Embryology The inner ear semicircular canals of bony labyrinth vestibule vestibulocochlear nerve cochlea middle ear auditory tube Location: In the petrous part of the temporal bone. Functions: Parts: • hearing (cochlea, spiral organ of Corti) • bony labyrinth • balance • membranous labyrinth • spacial orientation • ganglia and final branches of the vestibulocochlear nerve (VIII.) The bony (osseous) labyrinth vestibule semicircular canals cochlear branch of semicircular cochlea oval (vestibular) of bony labyrinth nerve VIII. canals window sagittal α frontal ∙ α ~45° α vestibular vestibulocochlear branch of nerve VIII. nerve (VIII.) round (cochlear) internal vestibule window cochlea acustic meatus promontory (base of the cochlea, medial wall of the tympanic cavity) Left osseous labyrinth, lateral view Bony limbs (crura) of semicircular canals (5): superior (vestibular connections) • 3 ampullary crura ampullary osseus crura • 1 simple crus, common crus • 1 common crus elliptical recess spherical recess apex lateral (cupula) posterior simple crus cochlea fenestra vestibuli ampullary osseus crus base fenestra cochleae Interior of the vestibule Maculae cribrosae: • superior, media, inferior, • perforated areas for entering the branches of the vestibular elliptical recess nerve: ( for the utricle) 1. utriculoampullar nerve, spherical 2. saccular nerve, recess ( for the saccule) 3. posterior ampullary nerve semicircular canals -

Enlarged Cochlear Aqueduct
AJNR Am J Neuroradiol 19:330–332, February 1998 Enlarged Cochlear Aqueduct Suresh K. Mukherji, Henry C. Baggett, Jay Alley, and Vincent H. Carrasco Summary: Enlargement of the cochlear aqueduct is a con- mm. The right cochlear aqueduct was slightly irregular but not troversial topic, with experienced investigators doubting its definitely enlarged. existence because of a lack of published cases. We describe On the basis of clinical and audiologic examination, the the CT appearance of an enlarged cochlear aqueduct in a patient was considered to be a suitable candidate for cochlear implantation. Typically, the ear with the greatest hearing loss is patient with advanced congenital inner ear anomalies and implanted to prevent conflicting signals between the implant congenital hearing loss. The intent of this article is to and normal auditory signal transmission. At 2 years of age, the present the CT appearance of a presumably enlarged co- patient received a Clarion (Simi Valley, Calif) eight-channel chlear aqueduct and to underscore the need to examine this cochlear implant in the right ear. No perilymphatic fistula structure in patients with congenital hearing loss. developed during cochleotomy for insertion of the electrode. The patient did well postoperatively and early evaluation indi- cated that the implant was stimulating well. Three months after The cochlear aqueduct is a bony canal that connects the implantation, the patient had a 50-dB hearing threshold and subarachnoid space to the basal turn of the cochlea. Enlarge- conditioned responses to stimulation. ment of the cochlear aqueduct has been suggested as a cause of sensorineural hearing loss and perilymph fistula (1). -

A Chronology of Middle Missouri Plains Village Sites
Smithsonian Institution Scholarly Press smithsonian contributions to zoology • number 627 Smithsonian Institution Scholarly Press TheA Chronology Therian Skull of MiddleA Missouri Lexicon with Plains EmphasisVillage on the OdontocetesSites J. G. Mead and R. E. Fordyce By Craig M. Johnson with contributions by Stanley A. Ahler, Herbert Haas, and Georges Bonani SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of “diffusing knowledge” was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: “It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge.” This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, com- mencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Botany Smithsonian Contributions in History and Technology Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Museum Conservation Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology In these series, the Institution publishes small papers and full-scale monographs that report on the research and collections of its various museums and bureaus. The Smithsonian Contributions Series are distributed via mailing lists to libraries, universities, and similar institu- tions throughout the world. Manuscripts submitted for series publication are received by the Smithsonian Institution Scholarly Press from authors with direct affilia- tion with the various Smithsonian museums or bureaus and are subject to peer review and review for compliance with manuscript preparation guidelines.