Neuroanatomy Draw It to Know It 2Nd Edition (
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

INDEX Abducent Neurons Anatomy 135 Clinical Signs 137 Diseases
INDEX Abducent neurons Anatomy 135 Clinical signs 137 Diseases 139 Function 135 Abiotrophic sensorineural deafness 438 Abiotrophy 100, 363 Auditory 438 Cerebellar cortical 363 Motor neuron 100 Nucleus ambiguus 159 Peripheral vestibular 336 Abscess-Brainstem 330 Caudal cranial fossa 343 Cerebellar 344 Cerebral 416, 418 Pituitary 162 Streptococcus equi 418 Abyssian cat-Glucocerebrosidosis 427 Myastheina gravis 93 Accessory neurons Anatomy 152 Clinical signs 153 Diseases 153 Acetozolamide 212 Acetylcholine 78, 169, 354, 468, 469 Receptor 78 Acetylcholinesterase 79 Achiasmatic Belgian sheepdog 345 Acoustic stria 434 Acral mutilation 237 Adenohypophysis 483 Releasing factors 483 Adenosylmethionine 262 Adhesion-Interthalamic 33, 476 Adiposogenital syndrome 484 Adipsia 458, 484 Adrenocorticotrophic hormone 485 Adversive syndrome 72, 205, 460 Afghan hound-Inherited myelinolytic encephalomyelopathy 264 Agenized flour 452 Aino virus 44 Akabane virus 43 Akita-Congenital peripiheral vestibular disease 336 Alaskan husky encephalopathy 522 Albinism, ocular 345 Albinotic sensorineural deafness 438 Alexander disease 335 Allodynia 190 Alpha fucosidosis 427 Alpha glucosidosis 427 Alpha iduronidase 427 Alpha mannosidase 427 Alpha melanotropism 484 Alsatian-idiopathic epilepsy 458 Alternative anticonvulsant drugs 466 American Bulldog-Ceroid lipofuscinosis 385, 428 American Miniature Horse-Narcolepsy 470 American StaffordshireTerrier-Cerebellar cortical abiotrophy 367 Amikacin 329 Aminocaproic acid 262 Aminoglycoside antibiotics 329, 439 Amprolium toxicity -

Somatosensory Systems
Somatosensory Systems Sue Keirstead, Ph.D. Assistant Professor Dept. of Integrative Biology and Physiology Stem Cell Institute E-mail: [email protected] Tel: 612 626 2290 Class 9: Somatosensory System (p. 292-306) 1. Describe the 3 main types of somatic sensations: 1. tactile: light touch, deep pressure, vibration, cold, hot, etc., 2. pain, 3. Proprioception. 2. List the types of sensory receptors that are found in the skin (Figure 9.11) and explain what determines the optimum type of stimulus that will activate each. 3. Describe the two different modality-specific ascending somatosensory pathways and note which modalities are carried in each (Figure 9.10 and 9.13). 4. Describe how it is possible for us to differentiate between stimuli of different modalities in the same body part (i.e. fingertip). Consider this at the level of 1) the sensory receptors and 2) the neurons onto which they synapse in the ascending sensory systems. 5. Explain how one might determine the location of a spinal cord injury based on the modality of sensation that is lost and the region of the body (both the side of the body and body part) where sensation is lost (Figure 9.18). 6. Describe how incoming sensory inputs from primary sensory axons can be modified at the level of the spinal cord and relate this to the mechanism of action of some common pain medications (Figure 9-18). 7. Describe the homunculus and explain the significance of the size of the region of the somatosensory cortex devoted to a particular body part. Cerebral cortex Interneuron Thalamus Interneuron 4 Integration of sensory Stimulus input in the CNS 1 Stimulation Sensory Axon of sensory of sensory receptor neuron receptor Graded potential Action potentials 2 Transduction 3 Generation of of the stimulus action potentials Copyright © 2016 by John Wiley & Sons, Inc. -

Proprioceptive- Deep Tendon Reflex Dr. Jose Palomar
PROPRIOCEPTIVE- DEEP TENDON REFLEX DR. JOSE PALOMAR PROPRIOCEPTIVE- DEEP TENDON REFLEX DR. JOSE PALOMAR Proprioceptive-Deep Tendon Reflex About the Author Dr. Jose Palomar Lever, M.D. is a native of Guadalajara, the capital city of the state of Jalisco in Mexico. He began his medical school education at the age of 17 at the Universidad Autónoma de Guadalajara (UAG) and received his training in Orthopedic Surgery and Traumatology at the Universidad del Ejercito y Fuerza Aérea (UDEFA). He performed his first orthopedic surgery at the age of 24 and between 1984 and 1988 was an orthopedic surgeon on the staff of the Reconstructive and Plastic Surgery Institute of Jalisco, S.S.A. He went on to receive specialized training in minimally invasive spine surgery at the Texas Back Institute in Dallas, Texas. Pursuing his interest in what he now refers to as the “software” of the human body, a study, which began in earnest for him in 2000, Dr. Palomar became a Diplomate in Applied Kinesiology from the International College of Applied Kinesiology (ICAK). He received the organization's Alan Beardall Memorial Award for Research for 2004-2005 and over the years has had eighteen papers accepted for inclusion in ICAK-USA Proceedings. He also completed the Carrick Institute for Graduate Studies program in Clinical Neurology. Today, in addition to pursuing an ongoing research program, Dr. Palomar conducts regular trainings in Proprioceptive-Deep Tendon Reflex (P-DTR) for medical practitioners in USA, Canada, Australia, Mexico, England, Poland, Latvia and Russia, and continues to practice medicine from his home base in Guadalajara, Mexico. -

High-Resolution Cortical Imaging
High-Resolution Cortical Imaging Alex de Crespigny Ph.D., Ellen Grant M.D., Larry Wald Ph.D., Jean Augustinack Ph.D., Bruce Fischl Ph.D., Helen D’Arceuil Ph.D. Martinos Center, Department of Radiology, Massachusetts General Hospital, Charlestown, MA Structure of the cortex The adult cerebral cortex is a complex convoluted multilayered structure averaging around 2.3mm thick, but thinner (≤ 2mm) in the depths of the sulcal folds and thicker (3-4mm) at the crown of the gyri. It is typically divided into 6 layers depending upon the constituent cell types. On examination a section of the cortex, it is seen to consist of alternating white and gray layers from the surface inward: (1) a thin layer of white substance; (2) a layer of gray substance; (3) a second white layer (outer band of Baillarger or band of Gennari); (4) a second gray layer; (5) a third white layer (inner band of Baillarger); (6) a third gray layer, which rests on the medullary substance of the gyrus (1). Cortical neurons form a highly organized laminar and radial structure with extensive efferents. Long association fibers connect to distant regions of the ipsilateral hemisphere, while short fibers connect to nearby ipsilateral regions. Commissural fibers connect to the cortical regions of the contralateral hemisphere, and projection fibers reach from the cortex into the subcortical structures e.g., corticothalamic/subthalamic projections, etc. High resolution structural MRI of the cortex Conventional diagnostic clinical MRI scans, with in plane resolution of ~1mm and slice thickness up to 5mm can define the gray-white interface of the cerebral cortex but cannot visualize structure within the cortex itself. -

A Hypothesis for the Evolution of the Upper Layers of the Neocortex Through Co-Option of the Olfactory Cortex Developmental Program
HYPOTHESIS AND THEORY published: 12 May 2015 doi: 10.3389/fnins.2015.00162 A hypothesis for the evolution of the upper layers of the neocortex through co-option of the olfactory cortex developmental program Federico Luzzati 1, 2* 1 Department of Life Sciences and Systems Biology (DBIOS), University of Turin, Turin, Italy, 2 Neuroscience Institute Cavalieri Ottolenghi, Orbassano, Truin, Italy The neocortex is unique to mammals and its evolutionary origin is still highly debated. The neocortex is generated by the dorsal pallium ventricular zone, a germinative domain that in reptiles give rise to the dorsal cortex. Whether this latter allocortical structure Edited by: contains homologs of all neocortical cell types it is unclear. Recently we described a Francisco Aboitiz, + + Pontificia Universidad Catolica de population of DCX /Tbr1 cells that is specifically associated with the layer II of higher Chile, Chile order areas of both the neocortex and of the more evolutionary conserved piriform Reviewed by: cortex. In a reptile similar cells are present in the layer II of the olfactory cortex and Loreta Medina, the DVR but not in the dorsal cortex. These data are consistent with the proposal that Universidad de Lleida, Spain Gordon M. Shepherd, the reptilian dorsal cortex is homologous only to the deep layers of the neocortex while Yale University School of Medicine, the upper layers are a mammalian innovation. Based on our observations we extended USA Fernando Garcia-Moreno, these ideas by hypothesizing that this innovation was obtained by co-opting a lateral University of Oxford, UK and/or ventral pallium developmental program. Interestingly, an analysis in the Allen brain *Correspondence: atlas revealed a striking similarity in gene expression between neocortical layers II/III Federico Luzzati, and piriform cortex. -
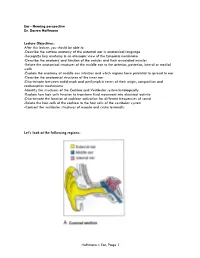
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Tissue Engineered Myelination and the Stretch Reflex Arc Sensory Circuit: Defined Medium Ormulation,F Interface Design and Microfabrication
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2009 Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium ormulation,F Interface Design And Microfabrication John Rumsey University of Central Florida Part of the Biology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Rumsey, John, "Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium Formulation, Interface Design And Microfabrication" (2009). Electronic Theses and Dissertations, 2004-2019. 3826. https://stars.library.ucf.edu/etd/3826 TISSUE ENGINEERED MYELINATION AND THE STRETCH REFLEX ARC SENSORY CIRCUIT: DEFINED MEDIUM FORMULATION, INTERFACE DESIGN AND MICROFABRICATION by JOHN WAYNE RUMSEY B.S. University of Florida, 2001 M.S. University of Central Florida, 2004 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Burnett School of Biomedical Sciences in the College of Medicine at the University of Central Florida Orlando, Florida Fall Term 2009 Major Professor: James J. Hickman ABSTRACT The overall focus of this research project was to develop an in vitro tissue- engineered system that accurately reproduced the physiology of the sensory elements of the stretch reflex arc as well as engineer the myelination of neurons in the systems. In order to achieve this goal we hypothesized that myelinating culture systems, intrafusal muscle fibers and the sensory circuit of the stretch reflex arc could be bioengineered using serum-free medium formulations, growth substrate interface design and microfabrication technology. -

The Structural Model: a Theory Linking Connections, Plasticity, Pathology, Development and Evolution of the Cerebral Cortex
Brain Structure and Function https://doi.org/10.1007/s00429-019-01841-9 REVIEW The Structural Model: a theory linking connections, plasticity, pathology, development and evolution of the cerebral cortex Miguel Ángel García‑Cabezas1 · Basilis Zikopoulos2,3 · Helen Barbas1,3 Received: 11 October 2018 / Accepted: 29 January 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The classical theory of cortical systematic variation has been independently described in reptiles, monotremes, marsupials and placental mammals, including primates, suggesting a common bauplan in the evolution of the cortex. The Structural Model is based on the systematic variation of the cortex and is a platform for advancing testable hypotheses about cortical organization and function across species, including humans. The Structural Model captures the overall laminar structure of areas by dividing the cortical architectonic continuum into discrete categories (cortical types), which can be used to test hypotheses about cortical organization. By type, the phylogenetically ancient limbic cortices—which form a ring at the base of the cerebral hemisphere—are agranular if they lack layer IV, or dysgranular if they have an incipient granular layer IV. Beyond the dysgranular areas, eulaminate type cortices have six layers. The number and laminar elaboration of eulaminate areas differ depending on species or cortical system within a species. The construct of cortical type retains the topology of the systematic variation of the cortex and forms the basis for a predictive Structural Model, which has successfully linked cortical variation to the laminar pattern and strength of cortical connections, the continuum of plasticity and stability of areas, the regularities in the distribution of classical and novel markers, and the preferential vulnerability of limbic areas to neurodegenerative and psychiatric diseases. -

BRS Physiology 3Rd Edition
Board Review Series • Reflects USMLE changes • Approximately 350 USMLE-type questions with explanations • Numerous illustrations, diagrams, and tables • Easy-to-follow outline covering all USMLE-tested topics • A comprehensive examination V Ah, LIPPINCOTT -"*" WILLIAMS &WILKIN; mum IEEE mows IF 'IMP IMMO MINK I I. Key Physiology Topics for USMLE Step I Cell Physiology Transport mechanisms Ionic basis for action potential Excitation-contraction coupling in skeletal, cardiac, and smooth muscle Neuromuscular transmission Autonomic Physiology Cholinergic receptors Adrenergic receptors Effects of autonomic nervous system on organ system function Cardiovascular Physiology Events of cardiac cycle Pressure, flow, resistance relationships Frank-Starling law of the heart Ventricular pressure-volume loops Ionic basis for cardiac action potentials Starling forces in capillaries Regulation of arterial pressure (baroreceptors and renin-angiotensin II-aldosterone system) Cardiovascular and pulmonary responses to exercise Cardiovascular responses to hemorrhage Cardiovascular responses to changes in posture Respiratory Physiology Lung and chest-wall compliance curves Breathing cycle Hemoglobin-02 dissociation curve Causes of hypoxemia and hypoxia vq, P02, and P00 2 in upright lung V/Q defects Peripheral and central chemoreceptors in control of breathing Responses to high altitude Renal and Acid-Base Physiology Fluid shifts between body fluid compartments Starling forces across glomerular capillaries Transporters in various segments of nephron (Na Cl-, -

Full Disclosures
RESIDENT & FELLOW SECTION Clinical Reasoning: Section Editor An unusual case of subacute Mitchell S.V. Elkind, MD, MS encephalopathy Neal Parikh, MD SECTION 1 revealed wide-based gait and lower extremity dysmet- Alexander E. Merkler, A 52-year-old previously healthy man presented with 8 ria. Relevant laboratory evaluation revealed only a MD months of progressive cognitive decline. He complained C-reactive protein of 1.79 (normal 0–0.99). Natalie T. Cheng, MD of months of confusion, fatigue, depression, hypersom- Brain MRI obtained in Morocco 2 months prior Hediyeh Baradaran, MD nolence, headaches, and, subsequently, urinary inconti- to presentation had demonstrated bilateral thalamic Halina White, MD nence and unsteady gait. His family reported that he T2 hyperintensities and patchy enhancement in the Dana Leifer, MD spoke of his deceased mother as if she were alive. His right medial temporal lobe, midbrain, and basal gan- executive deficits progressed, leading to termination of glia. A right thalamic biopsy obtained in Morocco his employment and a motor vehicle accident. He had revealed “inflammatory cells” without evidence Correspondence to was evaluated and treated in Morocco before presenting of malignancy. Dr. Leifer: to our institution for further care. [email protected] Questions for consideration: Mental status examination was notable for slowed mentation and dyscalculia but was otherwise normal. 1. How can thalamic injury cause encephalopathy? Motor, sensory, and deep tendon reflex examination 2. What is the differential diagnosis for bilateral tha- results were normal. Cerebellar and gait examination lamic MRI abnormalities? GO TO SECTION 2 From the Departments of Neurology (N.P., A.E.M., N.T.C., H.W., D.L.) and Radiology (H.B.), Weill Cornell Medical Center, New York, NY. -

Management of Vertical Gaze Paresis from an Artery of Percheron Infarction
Abstract title: Management of Vertical Gaze Paresis from an Artery of Percheron Infarction. Abstract: A patient presents with vertical gaze paresis (down gaze > up gaze), a ‘drunk’ feeling when in a visually stimulating environment, speech impairment and memory impairment all stemming from a recent Artery of Percheron Infarction. Case History 40 year old white male Chief vision complaints on July 15, 2016: o Vertical gaze difficulty down gaze > up gaze o ‘Drunk’ feeling when in a visually stimulating environment (supermarket, crowd) Eye exam from 2015 was unremarkable o No glasses Rx was given o Patient has never worn glasses before Patient currently on Plavix and Lipitor medications Patient medical history: unremarkable, no prior medical issues o After event patient had speech impairment and mild memory impairment Pertinent findings Recent diagnosis of Artery of Percheron infarction (May 29, 2016) o Magnetic Resonance Imaging/Magnetic Resonance Angiogram images are available for viewing Poor convergence (27 cm) o Poor convergence even when adjusted for age Very low amplitude of accommodation for age: 2 D o Avg Amps for 40 yo = 5 D o Min Amps for 40 yo = 1.67 D Restricted down gaze > up gaze on EOM testing BCVA 20/20 OD/OS o Low Hyperopic Rx found o Minimal reading Rx found o Patient appreciated better vision with trial of glasses with manifest Rx Mild Meibomian Gland Dysfunction Posterior: unremarkable Blood work was negative except mildly elevated Protein S Differential diagnosis and reasons excluded Primary: ‘Top of the -

15 Telencephalon: Neocortex
15 Telencephalon: Neocortex Introduction.........................491 – Dendritic Clusters, Axonal Bundles and Radial Cell Cords As (Possible) Sulcal Pattern ........................498 Constituents of Neocortical Minicolumns . 582 Structural and Functional Subdivision – Microcircuitry of Neocortical Columns .... 586 ofNeocortex.........................498 – Neocortical Columns and Modules: – Structural Subdivision 1: Cytoarchitecture . 498 A Critical Commentary ............... 586 – Structural Subdivision 2: Myeloarchitecture . 506 Comparative Aspects ................... 591 – Structural Subdivision 3: Myelogenesis ....510 Synopsis of Main Neocortical Regions ...... 592 – Structural Subdivision 4: Connectivity .....510 – Introduction....................... 592 – Functional Subdivision ................516 – Association and Commissural Connections . 592 – Structural and Functional Subdivision: – Functional and Structural Asymmetry Overview .........................528 of the Two Hemispheres .............. 599 – Structural and Functional Localization – Occipital Lobe ..................... 600 in the Neocortex: Current Research – Parietal Lobe ...................... 605 and Perspectives ....................530 – TemporalLobe..................... 611 Neocortical Afferents ...................536 – Limbic Lobe and Paralimbic Belt ........ 617 Neocortical Neurons – FrontalLobe ...................... 620 and Their Synaptic Relationships ..........544 – Insula........................... 649 – IntroductoryNote...................544 – Typical Pyramidal