15 Telencephalon: Neocortex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

High-Resolution Cortical Imaging
High-Resolution Cortical Imaging Alex de Crespigny Ph.D., Ellen Grant M.D., Larry Wald Ph.D., Jean Augustinack Ph.D., Bruce Fischl Ph.D., Helen D’Arceuil Ph.D. Martinos Center, Department of Radiology, Massachusetts General Hospital, Charlestown, MA Structure of the cortex The adult cerebral cortex is a complex convoluted multilayered structure averaging around 2.3mm thick, but thinner (≤ 2mm) in the depths of the sulcal folds and thicker (3-4mm) at the crown of the gyri. It is typically divided into 6 layers depending upon the constituent cell types. On examination a section of the cortex, it is seen to consist of alternating white and gray layers from the surface inward: (1) a thin layer of white substance; (2) a layer of gray substance; (3) a second white layer (outer band of Baillarger or band of Gennari); (4) a second gray layer; (5) a third white layer (inner band of Baillarger); (6) a third gray layer, which rests on the medullary substance of the gyrus (1). Cortical neurons form a highly organized laminar and radial structure with extensive efferents. Long association fibers connect to distant regions of the ipsilateral hemisphere, while short fibers connect to nearby ipsilateral regions. Commissural fibers connect to the cortical regions of the contralateral hemisphere, and projection fibers reach from the cortex into the subcortical structures e.g., corticothalamic/subthalamic projections, etc. High resolution structural MRI of the cortex Conventional diagnostic clinical MRI scans, with in plane resolution of ~1mm and slice thickness up to 5mm can define the gray-white interface of the cerebral cortex but cannot visualize structure within the cortex itself. -

Imaging of Camp/PKA Dynamics Induced by Catecholamines in the Neocortical Network Shinobu Nomura
Imaging of cAMP/PKA dynamics induced by catecholamines in the neocortical network Shinobu Nomura To cite this version: Shinobu Nomura. Imaging of cAMP/PKA dynamics induced by catecholamines in the neocortical network. Neurons and Cognition [q-bio.NC]. Université Pierre et Marie Curie - Paris VI, 2014. English. NNT : 2014PA066440. tel-01374733 HAL Id: tel-01374733 https://tel.archives-ouvertes.fr/tel-01374733 Submitted on 1 Oct 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Thèse de doctorat de l’Université Pierre et Marie Curie, Paris 6 Spécialité : Neurosciences Ecole doctorale Cerveau-Cognition-Comportement (ED3C) Présentée par NOMURA Shinobu Pour obtenir le grade de Docteur de l’Université Paris 6 Sujet de la thèse : Imaging of cAMP/PKA dynamics induced by catecholamines in the neocortical network Soutenue le 26 septembre 2014 Devant le jury composé de : Dr Denis Hervé, Président Dr Emmanuel Valjent, Rapporteur Pr Philippe De Deurwaerdère, Rapporteur Dr Régine Hepp, Directeur de thèse Invités exceptionnels Dr Nicolas Gervasi Dr Thierry Gallopin Dr. Bertrand Lambolez 1 Remerciements Je voudrais tout d'abord remercier mon directeur de thèse, Bertrand Lambolez, pour m’avoira ccueilli pour mon stage de M2 puis de m’avoir proposé ce projet sur la transmission catécholaminergique pour poursuivre une thèse à l’UPMC. -

Target-Specific Contrast Agents for Magnetic Resonance Microscopy
NeuroImage 46 (2009) 382–393 Contents lists available at ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Target-specific contrast agents for magnetic resonance microscopy Megan L. Blackwell a,b,⁎, Christian T. Farrar a,d, Bruce Fischl a,c,d, Bruce R. Rosen a,d a Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, USA b Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA c Computer Science and Artificial Intelligence Laboratory, MIT, Cambridge, MA, USA d Department of Radiology, Massachusetts General Hospital, Charlestown, MA, USA article info abstract Article history: High-resolution ex vivo magnetic resonance (MR) imaging can be used to delineate prominent architectonic Received 2 July 2007 features in the human brain, but increased contrast is required to visualize more subtle distinctions. To aid Revised 5 December 2008 MR sensitivity to cell density and myelination, we have begun the development of target-specific Accepted 15 January 2009 paramagnetic contrast agents. This work details the first application of luxol fast blue (LFB), an optical Available online 30 January 2009 stain for myelin, as a white matter-selective MR contrast agent for human ex vivo brain tissue. Formalin-fixed human visual cortex was imaged with an isotropic resolution between 80 and 150 μm at 4.7 and 14 T before and after en bloc staining with LFB. Longitudinal (R1) and transverse (R2) relaxation rates in LFB-stained tissue increased proportionally with myelination at both field strengths. Changes in R1 resulted in larger contrast-to-noise ratios (CNR), per unit time, on T1-weighted images between more myelinated cortical layers (IV–VI) and adjacent, superficial layers (I–III) at both field strengths. -

Direct Visualization of the Perforant Pathway in the Human Brain with Ex Vivo Diffusion Tensor Imaging
ORIGINAL RESEARCH ARTICLE published: 28 May 2010 HUMAN NEUROSCIENCE doi: 10.3389/fnhum.2010.00042 Direct visualization of the perforant pathway in the human brain with ex vivo diffusion tensor imaging Jean C. Augustinack1*, Karl Helmer1, Kristen E. Huber1, Sita Kakunoori1, Lilla Zöllei1,2 and Bruce Fischl1,2 1 Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, USA 2 Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, Cambridge, MA, USA Edited by: Ex vivo magnetic resonance imaging yields high resolution images that reveal detailed cerebral Andreas Jeromin, Banyan Biomarkers, anatomy and explicit cytoarchitecture in the cerebral cortex, subcortical structures, and white USA matter in the human brain. Our data illustrate neuroanatomical correlates of limbic circuitry with Reviewed by: Konstantinos Arfanakis, Illinois Institute high resolution images at high field. In this report, we have studied ex vivo medial temporal of Technology, USA lobe samples in high resolution structural MRI and high resolution diffusion MRI. Structural and James Gee, University of Pennsylvania, diffusion MRIs were registered to each other and to histological sections stained for myelin for USA validation of the perforant pathway. We demonstrate probability maps and fiber tracking from Christopher Kroenke, Oregon Health and Science University, USA diffusion tensor data that allows the direct visualization of the perforant pathway. Although it *Correspondence: is not possible to validate the DTI data with invasive measures, results described here provide Jean Augustinack, Athinoula A. an additional line of evidence of the perforant pathway trajectory in the human brain and that Martinos Center for Biomedical the perforant pathway may cross the hippocampal sulcus. -

Staging of Alzheimer Disease-Associated Neurowbrillary Pathology Using Parayn Sections and Immunocytochemistry
Acta Neuropathol (2006) 112:389–404 DOI 10.1007/s00401-006-0127-z METHODS REPORT Staging of Alzheimer disease-associated neuroWbrillary pathology using paraYn sections and immunocytochemistry Heiko Braak · Irina AlafuzoV · Thomas Arzberger · Hans Kretzschmar · Kelly Del Tredici Received: 8 June 2006 / Revised: 21 July 2006 / Accepted: 21 July 2006 / Published online: 12 August 2006 © Springer-Verlag 2006 Abstract Assessment of Alzheimer’s disease (AD)- revised here by adapting tissue selection and process- related neuroWbrillary pathology requires a procedure ing to the needs of paraYn-embedded sections (5–15 m) that permits a suYcient diVerentiation between initial, and by introducing a robust immunoreaction (AT8) for intermediate, and late stages. The gradual deposition hyperphosphorylated tau protein that can be processed of a hyperphosphorylated tau protein within select on an automated basis. It is anticipated that this neuronal types in speciWc nuclei or areas is central to revised methodological protocol will enable a more the disease process. The staging of AD-related neuroW- uniform application of the staging procedure. brillary pathology originally described in 1991 was per- formed on unconventionally thick sections (100 m) Keywords Alzheimer’s disease · NeuroWbrillary using a modern silver technique and reXected the pro- changes · Immunocytochemistry · gress of the disease process based chieXy on the topo- Hyperphosphorylated tau protein · Neuropathologic graphic expansion of the lesions. To better meet the staging · Pretangles demands of routine laboratories this procedure is Introduction This study was made possible by funding from the German Research Council (Deutsche Forschungsgemeinschaft) and BrainNet Europe II (European Commission LSHM-CT-2004- The development of intraneuronal lesions at selec- 503039). -

MITOCW | MIT9 14S09 Lec36-Mp3
MITOCW | MIT9_14S09_lec36-mp3 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu. PROFESSOR: So this class, we will talk about some of the cell types in the neocortex and the way they're connected. We'll talk about different regions of neocortex, that is actually after it had experienced, in evolution, a lot of expansion, and we're just going to deal with mammalian cortex in small animals, like rodents, and human being. And then we'll talk a little bit about some of the major fibers in and out of the membrain. We've talked about those already, but I want to review them to make sure that they're in your mind. And then we'll get, at least, to an introduction of thalamocortical connections, which again, you've had some because when we talked about visual system, we talked about the genicular body rejection in the visual area, primary visual cortex, the auditory cortex. We talked about medial geniculate body, somatosensory has been talked about several times. We talked about the ventral nucleus. All right. So what are the two most commonly encountered classes in the cortical cells? The most characteristic cell type in the neocortex is the pyramidal cell, and this is what they look like. This is Brodel's simplification of the pyramidal cell that shows their major characteristics, a cell body with a sort of pyramidal shape in an apical dendrite that goes up towards the surface, and many of these cells send their apical dendrite all the way up to the surface where they arborize right up in layer one. -

Chandelier Cells Control Excessive Cortical Excitation: Characteristics of Whisker-Evoked Synaptic Responses of Layer 2/3 Nonpyramidal and Pyramidal Neurons
The Journal of Neuroscience, June 2, 2004 • 24(22):5101–5108 • 5101 Behavioral/Systems/Cognitive Chandelier Cells Control Excessive Cortical Excitation: Characteristics of Whisker-Evoked Synaptic Responses of Layer 2/3 Nonpyramidal and Pyramidal Neurons Yinghua Zhu,1 Ruth L. Stornetta,1 and J. Julius Zhu1,2,3 1Department of Pharmacology, University of Virginia School of Medicine, Charlottesville, Virginia 22908, 2Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, and 3Max Planck Institute for Medical Research, Department of Cell Physiology, Heidelberg D-69120, Germany Chandelier cells form inhibitory axo-axonic synapses on pyramidal neurons with their characteristic candlestick-like axonal terminals. The functional role of chandelier cells is still unclear, although the preferential loss of this cell type at epileptic loci suggests a role in epilepsy.Herewereportanexaminationofwhisker-andspontaneousactivity-evokedresponsesinchandeliercellsandotherfast-spiking nonpyramidal neurons and regular-spiking pyramidal neurons in layer 2/3 of the barrel cortex. Fast-spiking nonpyramidal neurons, including chandelier cells, basket cells, neurogliaform cells, double bouquet cells, net basket cells, bitufted cells, and regular-spiking pyramidal neurons all respond to stimulation of multiple whiskers on the contralateral face. Whisker stimulation, however, evokes small, delayed EPSPs preceded by an earlier IPSP and no action potentials in chandelier cells, different from other nonpyramidal and pyramidal neurons. In addition, chandelier cells display a larger receptive field with lower acuity than other fast-spiking nonpyramidal neurons and pyramidal neurons. Notably, simultaneous dual whole-cell in vivo recordings show that chandelier cells, which rarely fire action poten- tials spontaneously, fire more robustly than other types of cortical neurons when the overall cortical excitation increases. -
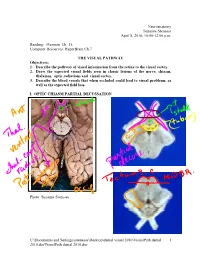
Visualpath Dental 2010.Jnt
Neuroanatomy Suzanne Stensaas April 8, 2010, 10:00-12:00 p.m. Reading: Waxman Ch. 15, Computer Resources: HyperBrain Ch 7 THE VISUAL PATHWAY Objectives: 1. Describe the pathway of visual information from the retina to the visual cortex. 2. Draw the expected visual fields seen in classic lesions of the nerve, chiasm, thalamus, optic radiations and visual cortex. 3. Describe the blood vessels that when occluded could lead to visual problems, as well as the expected field loss. I. OPTIC CHIASM PARTIAL DECUSSATION Photo: Suzanne Stensaas C:\Documents and Settings\sstensaas\Desktop\dental visual 2010\VisualPath dental 1 2010.docVisualPath dental 2010.doc II. OPTIC TRACT Ganglion cell axons diverge A. 90% go to Lateral geniculate nucleus (LGN) of thalamus (the retino- geniculo-calcarine path ) B. 10% go to Superior colliculus and pretectum (the retinocollicular path for reflexes) C. The hypothalamus for circadian rhythms (not to be discussed) III. THALAMIC RELAY NUCLEUS -- the LATERAL GENICULATE NUCLEUS OR BODY A. Specific retinotopic projection. B. Six layers. Three layers get input from from each eye. Thalamus Red LGN LGN Nucleus C:\Documents and Settings\sstensaas\Desktop\dental visual 2010\VisualPath dental 2 2010.docVisualPath dental 2010.doc The optic tract projects to the LGN Crainial Nerves, Wilson-Pauwels et al., 1988 IV. OPTIC RADIATIONS A. Retinotopic organization from the LGN neurons to the cortex. B. Axons of neurons in the lateral geniculate form the optic radiations = geniculocalcarine tract. The retinotopic organization is maintained. 1. Some loop forward over inferior (or temporal) horn of lateral ventricle = Meyer's Loop 2. Other axons take a more direct posterior course through the deep parietal white matter. -

NIH Public Access Author Manuscript Exp Brain Res
NIH Public Access Author Manuscript Exp Brain Res. Author manuscript; available in PMC 2013 September 30. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Exp Brain Res. 1998 December ; 123(3): 334–340. Co-localization of corticotropin-releasing hormone with glutamate decarboxylase and calcium-binding proteins in infant rat neocortical interneurons Xiao-Xin Yan, Tallie Z. Baram, Angelika Gerth, Linda Schultz, and Charles E. Ribak Department of Anatomy and Neurobiology, University of California at Irvine, Irvine, CA 92697, USA, Tel.: +1-714-824-5494, Fax: +1-714-824-8549 Charles E. Ribak: [email protected] Abstract Corticotropin releasing hormone (CRH) has been localized to interneurons of the mammalian cerebral cortex, but these neurons have not been fully characterized. The present study determined the extent of colocalization of CRH with glutamate decarboxylase (GAD) and calcium-binding proteins in the infant rat neocortex using immunocytochemistry. CRH-immunoreactive (ir) neurons were classified into two major groups. The first group was larger and consisted of densely CRH-immunostained small bipolar cells, predominantly localized to layers II and III. The second group of CRH-ir cells was lightly labeled and included multipolar neurons mainly found in deep cortical layers. Co-localization studies indicated that the vast majority of CRH-ir neurons, including both bipolar and multipolar types, was co-immunolabeled for GAD-65 and GAD-67. Most multipolar, but only some bipolar, CRH-ir neurons also contained parvalbumin, while CRH- ir neurons rarely contained calbindin or calretinin. These results indicate that virtually all CRH-ir neurons in the rat cerebral cortex are GABA-ergic. -

Staging of Alzheimer Disease-Associated Neurowbrillary Pathology Using Parayn Sections and Immunocytochemistry
CORE Metadata, citation and similar papers at core.ac.uk Provided by Springer - Publisher Connector Acta Neuropathol (2006) 112:389–404 DOI 10.1007/s00401-006-0127-z METHODS REPORT Staging of Alzheimer disease-associated neuroWbrillary pathology using paraYn sections and immunocytochemistry Heiko Braak · Irina AlafuzoV · Thomas Arzberger · Hans Kretzschmar · Kelly Del Tredici Received: 8 June 2006 / Revised: 21 July 2006 / Accepted: 21 July 2006 / Published online: 12 August 2006 © Springer-Verlag 2006 Abstract Assessment of Alzheimer’s disease (AD)- revised here by adapting tissue selection and process- related neuroWbrillary pathology requires a procedure ing to the needs of paraYn-embedded sections (5–15 m) that permits a suYcient diVerentiation between initial, and by introducing a robust immunoreaction (AT8) for intermediate, and late stages. The gradual deposition hyperphosphorylated tau protein that can be processed of a hyperphosphorylated tau protein within select on an automated basis. It is anticipated that this neuronal types in speciWc nuclei or areas is central to revised methodological protocol will enable a more the disease process. The staging of AD-related neuroW- uniform application of the staging procedure. brillary pathology originally described in 1991 was per- formed on unconventionally thick sections (100 m) Keywords Alzheimer’s disease · NeuroWbrillary using a modern silver technique and reXected the pro- changes · Immunocytochemistry · gress of the disease process based chieXy on the topo- Hyperphosphorylated tau protein · Neuropathologic graphic expansion of the lesions. To better meet the staging · Pretangles demands of routine laboratories this procedure is Introduction This study was made possible by funding from the German Research Council (Deutsche Forschungsgemeinschaft) and BrainNet Europe II (European Commission LSHM-CT-2004- The development of intraneuronal lesions at selec- 503039). -

Development, Diversity and Death of MGE-Derived Cortical Interneurons
International Journal of Molecular Sciences Review Development, Diversity, and Death of MGE-Derived Cortical Interneurons Rhîannan H. Williams 1 and Therese Riedemann 2,* 1 Helmholtz Zentrum München, German Research Centre for Environmental Health, Institute for Neurogenomics, Ingolstädter Landstraße 1, 85764 Neuherberg, Germany; [email protected] 2 Ludwig-Maximilians-Universität München, Biomedical Center, Physiological Genomics, Grosshaderner Str. 9, 82152 Planegg-Martinsried, Germany * Correspondence: [email protected] Abstract: In the mammalian brain, cortical interneurons (INs) are a highly diverse group of cells. A key neurophysiological question concerns how each class of INs contributes to cortical circuit function and whether specific roles can be attributed to a selective cell type. To address this question, researchers are integrating knowledge derived from transcriptomic, histological, electrophysiologi- cal, developmental, and functional experiments to extensively characterise the different classes of INs. Our hope is that such knowledge permits the selective targeting of cell types for therapeutic endeavours. This review will focus on two of the main types of INs, namely the parvalbumin (PV+) or somatostatin (SOM+)-containing cells, and summarise the research to date on these classes. Keywords: GABA; cortical interneurons; somatostatin; parvalbumin; interneuron diversity; interneuron development 1. Introduction Citation: Williams, R.H.; The cortex of the mammalian brain is composed of two main neuronal groups: pro- Riedemann, T. Development, jection neurons and interneurons (INs) [1–8]. Projection neurons are cells whose axons Diversity, and Death of MGE-Derived extend from the region where they are located to other brain areas and/or to the spinal Cortical Interneurons. Int. J. Mol. Sci. cord. -

Complete Dissertation Double
PARVALBUMIN-CONTAINING INTERNEURON CONNECTIVITY AND CRITICAL PERIOD MODULATION Marc Nahmani Rockville, MD B.S., University of Maryland, 1999 A Dissertation presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of Psychology University of Virginia August, 2008 Dr. Alev Erisir Dr. Peter Brunjes Dr. David Hill Dr. Cedric Williams Dr. Bar Condron 2 ABSTRACT Local inhibitory interneurons in cortex comprise a diverse group of cells whose properties may lend themselves to distinct roles within cortical microcircuits. Yet the position of these cells with respect to incoming sensory input, and the development of their specific laminar connections have not been established. Fast-spiking parvalbumin-containing (PV) cells comprise the largest subset of GABAergic interneurons in visual cortex, and have been implicated in developmental plasticity. To explore the position of these cells with respect to sensory input, I determined the synaptic contribution of thalamic input onto PV interneurons in layer 4 of ferret visual cortex at early postnatal and adult ages. Utilizing dual and triple-labeling immuno-electron microscopy to visualize thalamic arbors, PV interneurons and GABAergic cells, I determined that ~10% of all thalamocortical targets in adult layer 4 were PV-positive, and that every GABAergic target was immunoreactive for parvalbumin. This selective thalamocortical drive onto parvalbumin fast-spiking cells may account for the total thalamic innervation to GABAergic interneurons in adult layer 4. To investigate how PV interneuron inhibition impinges upon the subsequent cortical circuit, I conducted a laminar developmental analysis of PV terminal synapses with GABAergic and non-GABAergic targets using pre- and post-embedding electron microscopy.