A Non-Invasive Method for Monitoring Intracranial Pressure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

INDEX Abducent Neurons Anatomy 135 Clinical Signs 137 Diseases
INDEX Abducent neurons Anatomy 135 Clinical signs 137 Diseases 139 Function 135 Abiotrophic sensorineural deafness 438 Abiotrophy 100, 363 Auditory 438 Cerebellar cortical 363 Motor neuron 100 Nucleus ambiguus 159 Peripheral vestibular 336 Abscess-Brainstem 330 Caudal cranial fossa 343 Cerebellar 344 Cerebral 416, 418 Pituitary 162 Streptococcus equi 418 Abyssian cat-Glucocerebrosidosis 427 Myastheina gravis 93 Accessory neurons Anatomy 152 Clinical signs 153 Diseases 153 Acetozolamide 212 Acetylcholine 78, 169, 354, 468, 469 Receptor 78 Acetylcholinesterase 79 Achiasmatic Belgian sheepdog 345 Acoustic stria 434 Acral mutilation 237 Adenohypophysis 483 Releasing factors 483 Adenosylmethionine 262 Adhesion-Interthalamic 33, 476 Adiposogenital syndrome 484 Adipsia 458, 484 Adrenocorticotrophic hormone 485 Adversive syndrome 72, 205, 460 Afghan hound-Inherited myelinolytic encephalomyelopathy 264 Agenized flour 452 Aino virus 44 Akabane virus 43 Akita-Congenital peripiheral vestibular disease 336 Alaskan husky encephalopathy 522 Albinism, ocular 345 Albinotic sensorineural deafness 438 Alexander disease 335 Allodynia 190 Alpha fucosidosis 427 Alpha glucosidosis 427 Alpha iduronidase 427 Alpha mannosidase 427 Alpha melanotropism 484 Alsatian-idiopathic epilepsy 458 Alternative anticonvulsant drugs 466 American Bulldog-Ceroid lipofuscinosis 385, 428 American Miniature Horse-Narcolepsy 470 American StaffordshireTerrier-Cerebellar cortical abiotrophy 367 Amikacin 329 Aminocaproic acid 262 Aminoglycoside antibiotics 329, 439 Amprolium toxicity -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -
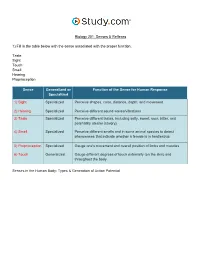
Senses & Reflexes in the Nervous System Visual Worksheet
Biology 201: Senses & Reflexes 1) Fill in the table below with the sense associated with the proper function. Taste Sight Touch Smell Hearing Proprioception Sense Generalized or Function of the Sense for Human Response Specialized 1) Sight Specialized Perceive shapes, color, distance, depth, and movement 2) Hearing Specialized Perceive different sound waves/vibrations 3) Taste Specialized Perceive different tastes, including salty, sweet, sour, bitter, and potentially umami (savory) 4) Smell Specialized Perceive different smells and in some animal species to detect pheromones that indicate whether a female is in heat/estrus 5) Proprioception Specialized Gauge one's movement and overall position of limbs and muscles 6) Touch Generalized Gauge different degrees of touch externally (on the skin) and throughout the body Senses in the Human Body: Types & Generation of Action Potential 2) Label the structures of the eye. Pupil Sclera Retina Lens Iris Cornea The Eye: Structure, Image Detection & Disorders 3) Label the chambers and structures of the eye below. Posterior chamber Muscle Retina Conjunctiva Cornea Sclera Optic nerve Anterior chamber Pupil Choroid layer Blood vessels Lens Iris Vitreous chamber The Eye: Structure, Image Detection & Disorders 4) Label the features of the visual pathway below. Optic chiasm Left cerebral hemisphere Pretectal nucleus Lateral geniculate nucleus of the thalamus Superior colliculus Visual cortex Right cerebral hemisphere The Eye: Structure, Image Detection & Disorders 5) Study the image of the section of the retina below. Label the neural layers. Bipolar cell Cone cell Neural layer Pigmented layer Rod cell Ganglion cell The Eye: Structure, Image Detection & Disorders 6) Label the structures of the nose below. -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Research Reports
ARAŞTIRMALAR (ResearchUnur, Ülger, Reports) Ekinci MORPHOMETRICAL AND MORPHOLOGICAL VARIATIONS OF MIDDLE EAR OSSICLES IN THE NEWBORN* Yeni doğanlarda orta kulak kemikciklerinin morfometrik ve morfolojik varyasyonları Erdoğan UNUR 1, Harun ÜLGER 1, Nihat EKİNCİ 2 Abstract Özet Purpose: Aim of this study was to investigate the Amaç: Yeni doğanlarda orta kulak kemikciklerinin morphometric and morphologic variations of middle ear morfometrik ve morfolojik varyasyonlarını ortaya ossicles. koymak. Materials and Methods: Middle ear of 20 newborn Gereç ve yöntem: Her iki cinse ait 20 yeni doğan cadavers from both sexes were dissected bilaterally and kadavrasının orta kulak boşluğuna girilerek elde edilen the ossicles were obtained to investigate their orta kulak kemikcikleri üzerinde morfometrik ve morphometric and morphologic characteristics. morfolojik inceleme yapıldı. Results: The average of morphometric parameters Bulgular: Morfometrik sonuçlar; malleus’un toplam showed that the malleus was 7.69 mm in total length with uzunluğu 7.69 mm, manibrium mallei’nin uzunluğu 4.70 an angle of 137 o; the manibrium mallei was 4.70 mm, mm, caput mallei ve processus lateralis arasındaki and the total length of head and neck was 4.85 mm; the uzaklık 4.85 mm, manibrium mallei’nin ekseni ve caput incus had a total length of 6.47 mm, total width of 4.88 mallei arasındaki açı 137 o, incus’un toplam uzunluğu mm , and a maximal distance of 6.12 mm between the 6.47 mm, toplam genişliği 4.88 mm, crus longum ve tops of the processes, with an angle of 99.9 o; the stapes breve’nin uçları arasındaki uzaklık 6.12 mm, cruslar had a total height of 3.22 mm, with stapedial base being arasındaki açı 99.9 o, stapesin toplam uzunluğu 2.57 mm in length and 1.29 mm in width. -

Ear Infections in Children
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Ear Infections in Children What is an ear infection? How can I tell if my child has an ear infection? An ear infection is an inflammation of the middle ear, usually caused by bacteria, that occurs when fluid builds Most ear infections happen to children before they’ve up behind the eardrum. Anyone can get an ear infection, learned how to talk. If your child isn’t old enough to say but children get them more often than adults. Five out of “My ear hurts,” here are a few things to look for: six children will have at least one ear infection by their third } Tugging or pulling at the ear(s) birthday. In fact, ear infections are the most common reason parents bring their child to a doctor. The scientific name for } Fussiness and crying an ear infection is otitis media (OM). } Trouble sleeping What are the symptoms of an } Fever (especially in infants and younger children) ear infection? } Fluid draining from the ear } Clumsiness or problems with balance There are three main types of ear infections. Each has a different combination of symptoms. } Trouble hearing or responding to quiet sounds. } Acute otitis media (AOM) is the most common ear What causes an ear infection? infection. Parts of the middle ear are infected and swollen and fluid is trapped behind the eardrum. This An ear infection usually is caused by bacteria and often causes pain in the ear—commonly called an earache. -

Topographical Anatomy and Morphometry of the Temporal Bone of the Macaque
Folia Morphol. Vol. 68, No. 1, pp. 13–22 Copyright © 2009 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl Topographical anatomy and morphometry of the temporal bone of the macaque J. Wysocki 1Clinic of Otolaryngology and Rehabilitation, II Medical Faculty, Warsaw Medical University, Poland, Kajetany, Nadarzyn, Poland 2Laboratory of Clinical Anatomy of the Head and Neck, Institute of Physiology and Pathology of Hearing, Poland, Kajetany, Nadarzyn, Poland [Received 7 July 2008; Accepted 10 October 2008] Based on the dissections of 24 bones of 12 macaques (Macaca mulatta), a systematic anatomical description was made and measurements of the cho- sen size parameters of the temporal bone as well as the skull were taken. Although there is a small mastoid process, the general arrangement of the macaque’s temporal bone structures is very close to that which is observed in humans. The main differences are a different model of pneumatisation and the presence of subarcuate fossa, which possesses considerable dimensions. The main air space in the middle ear is the mesotympanum, but there are also additional air cells: the epitympanic recess containing the head of malleus and body of incus, the mastoid cavity, and several air spaces on the floor of the tympanic cavity. The vicinity of the carotid canal is also very well pneuma- tised and the walls of the canal are very thin. The semicircular canals are relatively small, very regular in shape, and characterized by almost the same dimensions. The bony walls of the labyrinth are relatively thin. -

Download PDF Intratemporal Course of the Facial Nerve
Romanian Journal of Morphology and Embryology 2010, 51(2):243–248 ORIGINAL PAPER Intratemporal course of the facial nerve: morphological, topographic and morphometric features NICOLETA MĂRU1), A. C. CHEIŢĂ2), CARMEN AURELIA MOGOANTĂ3), B. PREJOIANU4) 1)Department of Anatomy, Faculty of Dental Medicine 2)PhD candidate, ENT specialist “Carol Davila” University of Medicine and Pharmacy, Bucharest 3)ENT resident, Emergency County Hospital, Craiova University of Medicine and Pharmacy of Craiova 4)Tehno Electro Medical Company, Bucharest Abstract The purpose of this study is to present some morphological and morphometric aspects of the facial nerve and especially of the tympanic and mastoid segments of this nerve. The authors follow up a mesoscopic study concerning the tract (length, angulation, width) of these segments and the anatomic relations with the important structures of the middle ear. At the same time, some anatomical variations which involve the canal of the facial nerve (dehiscences, tract deviation or other anatomical deviations) are presented. To evaluate the risk of the facial nerve injury during operations for chronic otitis media with or without cholesteatoma, stapedectomy in otosclerosis, exploratory tympanotomy, tympanoplasty, canaloplasty, osteomas surgery or other otologic surgery that involve facial nerve area. The intricate course of the facial nerve through the temporal bone is of vital concern to all otologic surgeons, since it often traverses the surgical field. Therefore, authors will review the course of the facial canal through the petrosal portion of the temporal bone from the internal auditory meatus to the stylomastoid foramen, paying particular attention to its relations to adjacent structures. Keywords: intratemporal part, facial nerve. -

Patient Information – Ear Surgery Instructions
The Oregon Clinic, Plaza ENT Division 5050 NE Hoyt #655, Portland, OR 97213 Phone: 5034882400 Fax: 5032310121 Patient Information – Ear Surgery Instructions Pre- and Post-operative Instructions for Ear Surgery (not including ear tubes) Before Surgery: Many ear surgeries involve manipulation of the eardrum (tympanic membrane), and some require the removal of bone to facilitate the treatment of your ear disease. As with any operation, infection, scarring, and blood clot formation (hematoma) are possible. The facial nerve is at risk for injury or temporary weakness during any ear surgery. Dizziness following surgery may be expected. Hearing loss or ringing in the ear (tinnitus) may be more pronounced. Taste disturbance is not uncommon in certain ear surgeries for a few weeks following surgery and, in a few instances, could be prolonged or permanent. An incision may be made behind your ear, on your earlobe, or behind the pointed cartilage in front of your ear (the tragus). These areas normally heal without problems or obvious scars. Hair around the ear may or may not be shaved. Flying is usually permitted one month after surgery. Swimming may be allowed six weeks after surgery, but check with your doctor first before resuming swimming or other water sports. If your work is not strenuous and depending upon the type of surgery you’ve had, you may return to work 3 to 4 days from the date of surgery. Generally, you will be seen about 2-3 weeks after surgery. This gives your eardrum time to heal before we see you back. Pre-operative Instructions: 1. -

1 Surgical Anatomy Alexander Rauchfuss
Chapter 1 1 1 Surgical Anatomy Alexander Rauchfuss The temporal bone presents a very complex anatomy. Therefore this overview is restricted to some major points from the viewpoint of surgical anatomy. For more detailed information see “Suggested Reading”. Thetemporalboneaccordingtoitsdevelopmentalanatomyisdivisibleinto four parts: the squamous, mastoid, petrous, and tympanic portions. Points of topographical reference on the lateral surface are the external acoustic meatus with its suprameatal spine, the temporal line, and the mastoid process. Thebaseofthezygomaextendsasacrestposteriorlyandslightlyupward, forming the supramastoid crest or temporal line. The temporal line as a land- mark corresponds to the base of the medial cranial fossa/tegmen tympani, which in most cases of surgery can easily be identified. In combination with the radiological anatomy in a Schüller view it allows adequate planning of the surgical approach to the antrum via the mastoid. All figures show the anatomy of a left ear. 2 1 Surgical Anatomy Figs. 1.1–1.5. Temporal bone and sigmoid sinus Fig. 1.1. Temporal bone. The degree of pneumatization is inconstant. The extent and arrangement of air cells varies considerably from a minimal air cell system in the surroundings of the antrum to involvement of most of the tempo- ral bone. Pneumatization usually begins in late fetal life, progressing until the end of childhood. The pneumatization process starts from the antrum. In most cases one can describe the topography of the cells as follows: periantral, sino- dural, perisinual, perifacial and mastoid tip cells. According to the extension of the cells, there is only one rule: the further from the antrum, the bigger the cells Fig. -

Basic Anatomy and Physiology of the Ear 11
1 BASIC ANATOMY AND PHYSIOLOGY OF THE EAR J. Irwin Introduction The ear is a small, complex series of interlinked structures that are involved in both maintenance of normal balance and the sense of hearing. In order to hear, the ear collects the sound waves that arrive as pressure changes in air and converts these into neurochemical impulses that travel along the cochlear- vestibular nerve to the brain. There are both active and passive mechanisms involved in this process.The prime function of the vestibular system is to detect and compensate for movement. This includes the ability to maintain optic fix- ation despite movement and to initiate muscle reflexes to maintain balance. For the purposes of describing structure and function the ear is usually split into four distinct parts. These are the outer ear, the middle ear and the audi- tory and vestibular parts of the inner ear (Figure 1.1). The outer ear This is sometimes known as the external ear and consists of the ear that is visible on the side of the head (the pinna), the external auditory meatus (ear hole) and the ear canal (external auditory canal) that leads to the eardrum (or tympanic membrane). The tympanic membrane has three layers and the outer layer is usually included as part of the outer ear. THE PINNA This is, for the most part, a piece of cartilage covered by skin (Figure 1.2). There is also a fatty earlobe in most people. The skin covering the cartilage is Infection and Hearing Impairment. Edited by V.E. Newton and P.J.