Fetal Membranes and Placentation in Chiroptera A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fetal Membrane Architecture, Aging and Inflammation in Pregnancy And
Placenta 79 (2019) 40–45 Contents lists available at ScienceDirect Placenta journal homepage: www.elsevier.com/locate/placenta Fetal membrane architecture, aging and inflammation in pregnancy and parturition T ∗ ∗∗ Ramkumar Menona, , Lauren S. Richardsona, Martha Lappasb,c, a Division of Maternal-Fetal Medicine & Perinatal Research, Department of Obstetrics & Gynecology, The University of Texas Medical Branch at Galveston, Galveston, TX, USA b Obstetrics, Nutrition and Endocrinology Group, Department of Obstetrics and Gynaecology, University of Melbourne, Mercy Hospital for Women, Heidelberg, Victoria, Australia c Department of Obstetrics and Gynaecology, University of Melbourne, Mercy Hospital for Women, Heidelberg, Victoria, Australia ARTICLE INFO ABSTRACT Keywords: Preterm birth is the single major cause of infant mortality. Short and long term outcomes for infants are often Fetal membranes worse in cases of preterm premature rupture of the fetal membranes (pPROM). Thus, increased knowledge of the Structure structure characteristics of fetal membranes as well as the mechanisms of membrane rupture are essential if we Senescence are to develop effective treatment strategies to prevent pPROM. In this review, we focus on the role of in- Inflammation flammation and senescence in fetal membrane biology. 1. Introduction 2. Fetal membrane growth and microfractures Human fetal membranes (placental membranes or amniochorionic Amnion and chorion takes distinct growth trajectory upon embry- membranes) is the innermost tissue layer that forms the intrauterine ogenesis [9]. The development of amnion and chorion begins with cavity [1,2]. Fetal membranes are comprised of amnion (innermost embryogenesis although they do not participate in the formation of the layer of the intraamniotic cavity) and chorion (fetal tissue connected to embryo or fetus [10–12]. -

Infections at the Maternal–Fetal Interface: an Overview of Pathogenesis and Defence
REVIEWS Infections at the maternal–fetal interface: an overview of pathogenesis and defence Christina J. Megli 1 ✉ and Carolyn B. Coyne 2 ✉ Abstract | Infections are a major threat to human reproductive health, and infections in pregnancy can cause prematurity or stillbirth, or can be vertically transmitted to the fetus leading to congenital infection and severe disease. The acronym ‘TORCH’ (Toxoplasma gondii, other, rubella virus, cytomegalovirus, herpes simplex virus) refers to pathogens directly associated with the development of congenital disease and includes diverse bacteria, viruses and parasites. The placenta restricts vertical transmission during pregnancy and has evolved robust mechanisms of microbial defence. However, microorganisms that cause congenital disease have likely evolved diverse mechanisms to bypass these defences. In this Review, we discuss how TORCH pathogens access the intra- amniotic space and overcome the placental defences that protect against microbial vertical transmission. Infections during pregnancy can be associated with pathogen mediated, placenta mediated and/or can be devastating consequences to the pregnant mother and through inflammation induced previable delivery. developing fetus. Vertical transmission, defined as There are also outcomes of congenital infections that infection of the fetus from the maternal host, is a major do not manifest until after delivery. These can include cause of morbidity and mortality in pregnancy. In some hearing loss, developmental delays and/or blindness as cases, bacterial, viral and parasitic infections induce detailed below. dire outcomes in the fetus. The sequelae of infections Inflammation initiated by an infection of the mater in pregnancy include teratogenic effects, which cause nal host is also a known cause of preterm labour and can congenital anomalies; growth restriction, stillbirth, mis result in previable delivery or sequelae of prematurity10 carriage and neonatal death; prematurity; and maternal with lifelong consequences to the neonate. -

Cynopterus Minutus, Minute Fruit Bat
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2019: T136423A21985433 Scope: Global Language: English Cynopterus minutus, Minute Fruit Bat Assessment by: Ruedas, L. & Suyanto, A. View on www.iucnredlist.org Citation: Ruedas, L. & Suyanto, A. 2019. Cynopterus minutus. The IUCN Red List of Threatened Species 2019: e.T136423A21985433. https://dx.doi.org/10.2305/IUCN.UK.2019- 3.RLTS.T136423A21985433.en Copyright: © 2019 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: Arizona State University; BirdLife International; Botanic Gardens Conservation International; Conservation International; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Chordata Mammalia Chiroptera Pteropodidae Taxon Name: Cynopterus minutus Miller, 1906 Common Name(s): • English: Minute Fruit Bat Taxonomic Notes: This may be a species complex. -

PRG2 and AQPEP Are Misexpressed in Fetal Membranes in Placenta Previa and Percreta Elisa T. Zhang1, Roberta L. Hannibal1,6, Keyl
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.14.248807; this version posted August 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 PRG2 and AQPEP are misexpressed in fetal membranes in placenta previa and percreta 2 3 Elisa T. Zhang1, Roberta L. Hannibal1,6, Keyla M. Badillo Rivera1,7, Janet H.T. Song1,8, Kelly 4 McGowan1, Xiaowei Zhu1,2, Gudrun Meinhardt3, Martin Knöfler3, Jürgen Pollheimer3, Alexander 5 E. Urban1,2, Ann K. Folkins4, Deirdre J. Lyell5, Julie C. Baker1,5* 6 7 1DepartMent of Genetics, Stanford University School of Medicine, Stanford, California, United 8 States of AMerica. 9 2DepartMent of Psychiatry and Behavioral Sciences, Stanford University School of 10 Medicine, Stanford, California, United States of AMerica. 11 3DepartMent of Obstetrics and Gynaecology, Reproductive Biology Unit, Medical University of 12 Vienna, Vienna, Austria. 13 4DepartMent of Pathology, Stanford University School of Medicine, Stanford, California, United 14 States of AMerica. 15 5DepartMent of Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, 16 California, United States of AMerica. 17 6Present address: Second GenoMe, Inc., Brisbane, California, United States of AMerica. 18 7Present address: Eversana Consulting, South San Francisco, California, United States of 19 AMerica. 20 8Present address: Division of Genetics and GenoMics, Boston Children’s Hospital, Harvard 21 Medical School, Boston, Massachusetts, United States of AMerica. 22 23 *Correspondence: [email protected] 24 300 Pasteur Dr. -
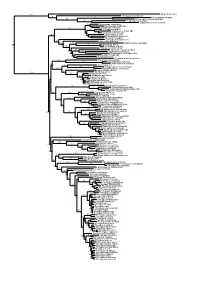
Figs1 ML Tree.Pdf
100 Megaderma lyra Rhinopoma hardwickei 71 100 Rhinolophus creaghi 100 Rhinolophus ferrumequinum 100 Hipposideros armiger Hipposideros commersoni 99 Megaerops ecaudatus 85 Megaerops niphanae 100 Megaerops kusnotoi 100 Cynopterus sphinx 98 Cynopterus horsfieldii 69 Cynopterus brachyotis 94 50 Ptenochirus minor 86 Ptenochirus wetmorei Ptenochirus jagori Dyacopterus spadiceus 99 Sphaerias blanfordi 99 97 Balionycteris maculata 100 Aethalops alecto 99 Aethalops aequalis Thoopterus nigrescens 97 Alionycteris paucidentata 33 99 Haplonycteris fischeri 29 Otopteropus cartilagonodus Latidens salimalii 43 88 Penthetor lucasi Chironax melanocephalus 90 Syconycteris australis 100 Macroglossus minimus 34 Macroglossus sobrinus 92 Boneia bidens 100 Harpyionycteris whiteheadi 69 Harpyionycteris celebensis Aproteles bulmerae 51 Dobsonia minor 100 100 80 Dobsonia inermis Dobsonia praedatrix 99 96 14 Dobsonia viridis Dobsonia peronii 47 Dobsonia pannietensis 56 Dobsonia moluccensis 29 Dobsonia anderseni 100 Scotonycteris zenkeri 100 Casinycteris ophiodon 87 Casinycteris campomaanensis Casinycteris argynnis 99 100 Eonycteris spelaea 100 Eonycteris major Eonycteris robusta 100 100 Rousettus amplexicaudatus 94 Rousettus spinalatus 99 Rousettus leschenaultii 100 Rousettus aegyptiacus 77 Rousettus madagascariensis 87 Rousettus obliviosus Stenonycteris lanosus 100 Megaloglossus woermanni 100 91 Megaloglossus azagnyi 22 Myonycteris angolensis 100 87 Myonycteris torquata 61 Myonycteris brachycephala 33 41 Myonycteris leptodon Myonycteris relicta 68 Plerotes anchietae -

Self-Organized Amniogenesis by Human Pluripotent Stem Cells in a Biomimetic Implantation-Like Niche
LETTERS PUBLISHED ONLINE: 12 DECEMBER 2016 | DOI: 10.1038/NMAT4829 Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche Yue Shao1†, Kenichiro Taniguchi2†, Katherine Gurdziel3, Ryan F. Townshend2, Xufeng Xue1, Koh Meng Aw Yong1, Jianming Sang1, Jason R. Spence2, Deborah L. Gumucio2* and Jianping Fu1,2,4* Amniogenesis—the development of amnion—is a critical factors seen in the in vivo amniogenic niche: a three-dimensional developmental milestone for early human embryogenesis (3D) extracellular matrix (ECM) that is provided by the basement and successful pregnancy1,2. However, human amniogenesis membrane surrounding the epiblast during implantation11; and a is poorly understood due to limited accessibility to peri- soft tissue bed provided by the uterine wall and trophoblast to implantation embryos and a lack of in vitro models. Here support the developing amnion (Fig. 1a,b). Since amniogenesis ini- we report an ecient biomaterial system to generate human tiates from the expanding pluripotent epiblast, we utilized mTeSR1 amnion-like tissue in vitro through self-organized development medium and basement membrane matrix (Geltrex) to render the of human pluripotent stem cells (hPSCs) in a bioengineered culture permissive for pluripotency maintenance. niche mimicking the in vivo implantation environment. We In this culture system, H9 human embryonic stem cells (hESCs) show that biophysical niche factors act as a switch to toggle were plated as single cells at 30,000 cells cm−2 onto a thick, hPSC self-renewal versus amniogenesis under self-renewal- soft gel bed of Geltrex (with thickness ≥100 µm, bulk Young's permissive biochemical conditions. We identify a unique modulus ∼900 Pa, coated on a glass coverslip), in mTeSR1 medium molecular signature of hPSC-derived amnion-like cells and supplemented with the ROCK inhibitor Y27632 (Fig. -

Increased Apoptosis in Chorionic Trophoblasts of Human Fetal Membranes with Labor at Term*
International Journal of Clinical Medicine, 2012, 3, 136-142 http://dx.doi.org/10.4236/ijcm.2012.32027 Published Online March 2012 (http://www.SciRP.org/journal/ijcm) Increased Apoptosis in Chorionic Trophoblasts of Human * Fetal Membranes with Labor at Term Hassan M. Harirah1#, Mostafa A. Borahay1, Wahidu Zaman1, Mahmoud S. Ahmed1, Ibrahim G. Hager2, Gary D. V. Hankins1 1The Department of Obstetrics & Gynecology, The University of Texas Medical Branch, Galveston, USA; 2The Department of Ob- stetrics & Gynecology, Zagazig University, Zagazig, Egypt. Email: #[email protected] Received December 7th, 2011; revised January 17th, 2012; accepted February 8th, 2012 ABSTRACT Objective: To determine the association of apoptosis in the layers of human fetal membranes distal to rupture site with labor at term. Study Design: Fetal membranes were collected from elective cesarean sections (n = 8) and spontaneous vaginal deliveries (n = 8) at term. The extent of apoptosis within fetal membrane layers was determined using terminal deoxynucleotidyl transferase deoxy-UTP-nick end labeling (TUNEL) assay and western blots for pro-apoptotic active caspase-3 and anti-apoptotic Bcl-2. Results: Apoptotic index in chorionic trophoblasts of membranes distal to rupture site obtained after vaginal delivery was 3-fold higher than those obtained from elective cesarean (11.57% ± 4.98% and 4.05% ± 2.4% respectively; p = 0.012). The choriodecidua layers after vaginal deliveries had higher expression of the pro-apoptotic active caspase-3 and less expression of the anti-apoptotic Bcl-2 than those obtained from elective cesar- ean sections. Conclusions: Labor at term is associated with increased apoptosis in chorionic trophoblast cells of human fetal membranes distal to rupture site. -

BMP-Treated Human Embryonic Stem Cells Transcriptionally Resemble Amnion Cells in the Monkey Embryo
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.21.427650; this version posted January 22, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. BMP-treated human embryonic stem cells transcriptionally resemble amnion cells in the monkey embryo Sapna Chhabra1,2,3, Aryeh Warmflash2,4* 1Systems Synthetic and Physical Biology graduate program, 2Department of Biosciences, 4Department of Bioengineering, Rice University, Houston, TX 77005 3Present address: Developmental Biology Unit, EMBL Heidelberg. *Correspondence to AW: [email protected] Abstract Human embryonic stem cells (hESCs) possess an immense potential to generate clinically relevant cell types and unveil mechanisms underlying early human development. However, using hESCs for discovery or translation requires accurately identifying differentiated cell types through comparison with their in vivo counterparts. Here, we set out to determine the identity of much debated BMP-treated hESCs by comparing their transcriptome to the recently published single cell transcriptomes of early human embryos in the study Xiang et al 2019. Our analyses reveal several discrepancies in the published human embryo dataset, including misclassification of putative amnion, intermediate and inner cell mass cells. These misclassifications primarily resulted from similarities in pseudogene expression, highlighting the need to carefully consider gene lists when making comparisons between cell types. In the absence of a relevant human dataset, we utilized the recently published single cell transcriptome of the early post implantation monkey embryo to discern the identity of BMP-treated hESCs. -

Bat Count 2003
BAT COUNT 2003 Working to promote the long term, sustainable conservation of globally threatened flying foxes in the Philippines, by developing baseline population information, increasing public awareness, and training students and protected area managers in field monitoring techniques. 1 A Terminal Report Submitted by Tammy Mildenstein1, Apolinario B. Cariño2, and Samuel Stier1 1Fish and Wildlife Biology, University of Montana, USA 2Silliman University and Mt. Talinis – Twin Lakes Federation of People’s Organizations, Diputado Extension, Sibulan, Negros Oriental, Philippines Photo by: Juan Pablo Moreiras 2 EXECUTIVE SUMMARY Large flying foxes in insular Southeast Asia are the most threatened of the Old World fruit bats due to deforestation, unregulated hunting, and little conservation commitment from local governments. Despite the fact they are globally endangered and play essential ecological roles in forest regeneration as seed dispersers and pollinators, there have been only a few studies on these bats that provide information useful to their conservation management. Our project aims to promote the conservation of large flying foxes in the Philippines by providing protected area managers with the training and the baseline information necessary to design and implement a long-term management plan for flying foxes. We focused our efforts on the globally endangered Philippine endemics, Acerodon jubatus and Acerodon leucotis, and the bats that commonly roost with them, Pteropus hypomelanus, P. vampyrus lanensis, and P. pumilus which are thought to be declining in the Philippines. Local participation is an integral part of our project. We conducted the first national training workshop on flying fox population counts and conservation at the Subic Bay area. -

The Science of Amnioexcite™ Three Layer Placental Membrane Allograft
The Science of AmnioExcite™ Three Layer Placental Membrane Allograft REV. 10-2020 The Science of AmnioExcite™ Placental Membrane Allograft AmnioExcite™ is a full-thickness decellularized placental membrane. AmnioExcite™ is a lyophilized, full-thickness placental membrane allograft decellularized with LifeNet Health’s proprietary Matracell® process and patent pending technology and intended for homologous use as a barrier membrane.(1) Inclusion of the intact amniotic and chorionic membranes, as well as the trophoblast layer, makes it thicker than most available amniotic-only or amniotic-chorionic allografts, and provides a robust protective covering while also delivering superior handling. AmnioExcite™ retains the placental membrane’s naturally occurring growth factors, cytokines, protease inhibitors, and extracellular matrix components, such as proteoglycans, collagen and fibronectin(2) In vitro studies have shown that these endogenous factors are capable of inducing cellular proliferation and migration, mitigating inflammation, and inhibiting protein degradation(3-5) STRUCTURE OF THE THREE LAYER PLACENTAL MEMBRANE AMNIOTIC MEMBRANE CHORIONIC MEMBRANE TROPHOBLAST LAYER The placental membrane is comprised of the amnion and chorion (6). The amnion, also called amniotic membrane (AM) has five layers, including the epithelium, basement membrane, compact layer, fibroblast layer, and the spongy layer(6), which provide important extracellular membrane components, as well as a wide variety of growth factors, cytokines, and other proteins.(7) While these characteristics are important, the AM by itself lacks substantial structure for providing a protective covering and contains only a small portion of the biological factors found in the full-thickness placental membrane. AM-only grafts can also be difficult to apply and may migrate away from the intended site of application.(8) The chorion is comprised of four layers, including the cellular layer, reticular layer, the pseudobasement membrane and the trophoblast layer (TL) (6). -

Attacked from Above and Below, New Observations of Cooperative and Solitary Predators on Roosting Cave Bats
bioRxiv preprint doi: https://doi.org/10.1101/550582; this version posted February 17, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Attacked from above and below, new observations of cooperative and solitary predators on roosting cave bats Krizler Cejuela. Tanalgo1, 2, 3, 7*, Dave L. Waldien4, 5, 6, Norma Monfort6, Alice Catherine Hughes1, 3* 1Landscape Ecology Group, Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 2International College, University of Chinese Academy of Sciences, Beijing 100049, People’s Republic of China 3Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 4Harrison Institute, Bowerwood House, 15 St Botolph’s Road, Sevenoaks, Kent, TN13 3AQ, United Kingdom 5Christopher Newport University, 1 Avenue of the Arts, Newport News, VA 23606, United States of America 6Philippine Bats for Peace Foundation Inc., 5 Ramona Townhomes, Guadalupe Village, Lanang, Davao City 7Department of Biological Sciences, College of Arts and Sciences, University of Southern Mindanao, Kabacan 9407, North Cotabato, the Republic of the Philippines Corresponding authors: ACH ([email protected]) and KCT ([email protected]) Landscape Ecology Group, Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China Abstract Predation of bats in their roosts has previously only been attributed to a limited number of species such as various raptors, owls, and snakes. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A.