Flavored Olive Oils: Focus on Their Acceptability and Thermal Stability
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Man Up: Integrating Fatherhood and Community Engagement, P. 15 Journal of Community Engagement and Scholarship Dr
Nonprofit Organization US Postage Paid Tuscaloosa AL Permit 16 The University of Alabama Office of Community Affairs — SCHOLARSHIP AND OF COMMUNITY ENGAGEMENT JOURNAL Vol. 4, No. 1, Spring 2011 Center for Community-Based Partnerships Box 870372 Tuscaloosa, AL 35487-0372 UGA and Tunisia: A Decade-Long Partnership, p. 5 Evaluating a Social Economy Research Partnership, p. 25 Teachinging RReeseasearch Dedication to Volume 4, No. 1, Spring 2011 4, No. Volume the Public Good: A Conundrum? Partnership p. 37 An International, FFaaccultyullty Students Indigenous Community Collaborative Inquiry, p. 50 The Journal of Community Engagement and Scholarship is published at The University of Alabama by the Office of Book Reviews, Community Affairs for the advancement p. 75 of engagement scholarship worldwide. Man Up: Integrating Fatherhood and Community Engagement, p. 15 Journal of Community Engagement and Scholarship Dr. Heather Pleasants, who is also book review editor of JCES, made the closing remarks at this year’s engaged scholarship awards program at The University of Alabama. Her words were based on the theme www.jces.ua.edu for the National Outreach Scholarship Conference (NOSC) that we will host in 2012. www.uapress.ua.edu Dr. Samory T. Pruitt, Vice President for Community Affairs, The University of Alabama Published twice a year by the Office of Community Affairs ach spring at The University of Alabama, we publicly recognize and celebrate at The University of Alabama the engaged scholarship that best represents our commitment to touching lives Distributed by The University of Alabama Press Ethrough community-based projects. ISSN: 1944-1207 We honor this work because of three important attributes at the foundation of engaged ISBN: 978-0-8173-7406-8 (paper) scholarship. -

Curriculum Vitae
Curriculum Vitae Personal Data Family name: Triki First name: Nesrine Sex: Female Date/Place of Birth: 13 March 1980/ Tunis – Tunisia Nationality: Tunisian Marital Status: Married. Home address: Ben Arous, Tunisia Work address: Institut Supérieur des Langues Appliquées et d'Informatique de Nabeul Av. Habib Thameur - 8000 Nabeul Tél.: (216) 72 230 551/ (216) 72 236 143- Fax : (216) 72 231 739 Mobile: (+216) 21914543 Emails: [email protected] Education . 2009- to the present: Doctoral studies, Faculty of Letters and Humanities Sfax (University of Sfax) Tunisia. Title: “Metadiscourse Across Genre Boundaries: Research Articles and PhD Theses Dissertations as a Case Study”. Supervisor: Professor Akila Assalemi- Baklouti . 2003-2006: MA Studies, Faculty of Letters, University of Mannouba, Department of English. Title: “Narratorial Techniques In Tunisian Police And Court Transcripts: A Forensic Linguistic Approach.” Supervisor: Professor: Mounir Triki . 1999-2003: BA (Maitrise), Faculty of Letters and Humanities Sfax (University of Sfax), Department of English. Memoir: “Non-Native Medical Abstract: A genre Approach”. Supervisor: Professor: Akila Assalemi-Baklouti Professional experience . 2011 to the present: Assistant Teacher at ISLAIN. Courses taught: Grammar/Key business concepts/Reading and Writing . 2004 -2011: (Teacher of English (Professeur Tronc Commun)): English Language Teaching: (English for Science and Technology) at Institut Supérieur d’Informatique et des Techniques de Communications, Hammam Sousse. Sousse, Tunisia. Co-ordinator and Responsible for the design, preparation and selection of course material for undergraduate students (DUT), (LMD) and Engineering Studies (DNI). 2008: (1 term): Higher Institute of Languages Applied to Business and Tourism, Moknine. Teaching Discourse Analysis module to Undergraduate Students. 2005-2007: English for Tourism: Reception and Travel Agency: IKET Elkantawi, Sousse . -

Download This PDF File
JANUARY -MARCH 2017 International Journal of Humanities and Cultural Studies ISSN 2356-5926 Editor-in-Chief: Dr. Hassen ZRIBA Volume 3 Issue 4 WWW. IJHCS . COM Volume 3 Issue 4 INTERNATIONAL JOURNAL OF HUMANITIES AND March 2017 CULTURAL STUDIES ISSN 2356-5926 Editorial Board Editor-in-Chief Managing Editor Dr. Hassen Zriba Najoua Chalbi *Emeritus Professor Ralph Grillo *Dr. Syed Zamanat Abbas University of Sussex, UK Salman Bin Abdulaziz University, Saudi Arabia *Professor Muhammad Asif *Dr. Santosh Kumar Behera Riphah International University, Pakistan Sidho-Kanho-Birsha University, India *Professor Sadok Bouhlila *Dr. P. Prayer Elmo Raj Northern Borders University, Saudi Arabia Pachaiyappa's College, India *Professor Pacha Malyadri *Omid Akhavan Osmania University, Andhra Pradesh, India Imam Ali University, Iran *Haron Bouras *Loredana Terec-Vlad Mohamed Cherif Messadia University, Souk- Ștefan cel Mare University, Romania Ahras Algeria *Professor Jason L. Powell *Dr. Shama Adams University of Chester, UK Curtin University, Australia *Professor Ali H. Raddaoui *Mansour Amini University of Wyoming, USA The Gulf College, Oman *Dr. Mohamed El-Kamel Bakari *Mohd AB Malek Bin MD Shah University of King Abdulaziz, Saudi Arabia Universiti Teknologi Mara, Malaysia *Dr. Solange Barros *Mark B. Ulla Federal University of Mato Grosso, Brazil Father Saturnino Urios University, Philipinnes *Dr. Salah Belhassen * Anouar Bennani University of Gafsa, Tunisia University of Sfax, Tunisia *Dr. Nodhar Hammami Ben Fradj *Shuv Raj Rana Bhat University of Kairouan, Tunisia Central Department of English Kirtipur, Kathmandu, Nepal *Dr. Arbind Kumar Choudhary *Erika Ashley Couto Rangachahi College, Majuli ,Assam, India University of Concordia, Canada *Dr. Amitabh Vikram Dwivedi *Md. Amir Hossain University of Shri Mata Vaishno Devi, India IBAIS University, Bangladesh *Dr. -

Sagesse Amélioration De La Gouvernance Dans Le Système De L’Enseignement Supérieur En Tunisie
sAGESSE Amélioration de la Gouvernance dans le système de l’EnSeignement Supérieur en Tunisie Objectives: * To contribute to the modernisation of the HE management and to the improvement of governance in Tunisia, providing an operational support methodologically rigorous and based on international best practices. * To create an integrated definition and control system for quality in university education in every university of the consortium. * To support the creation of new decision and management procedures to define the internal/external staff of the HEIs charged of the management and control of processes. * Development of skills and competencies by academic, administrative and managerial staff. * To promote the creation of an e-administration department in every university of the consortium for an innovative, effective and more autonomous management. Activities: * Research and analysis about best practices concerning governance in Tunisia. * Training seminars, study visits and best practice exchange to train the administrative and managerial staff. * Design of a Quality Handbook about financial management procedures. * In each university of the consortium: definition of a strategical project and related Action Plans and creation of offices to put them in place. * Information seminars about good governance addressed to the Tunisian Ministry for Higher Education and Scientific Research and the Tunisian National Body for Evaluation, Quality Assurance and Accreditation. * Elaboration of a White Book of recommendation addressed to interest groups and national, regional and international authorities. Follow the project IN ACTION Results: * Development of skills about governance and quality assessment by managerial and academic staff of the universities. * Development of skills about financial management by administrative staff of the universities. -

Albania Armenia Azerbaijan
ALBANIA University of Vlora “Ismail Qemali” (UV) https://univlora.edu.al/wp-content/uploads/2021/02/List-of-courses-2020-2021.pdf Polytechnic University of Tirana (PUoT) http://www.upt.al/en/faculties-and-institutes ARMENIA American University of Armenia (AUA) https://aua.am/academics/ Yerevan Brusov State University of Languages and Social Sciences https://brusov.am/en/science_list/bachelor_s_degree/ https://brusov.am/en/page_list/internationalapplicant/#sthash.B7eL5hIO.zGVzFc78.dpbs https://brusov.am/en/faculty_list/faculties/ Armenian State University of Economics https://asue.am/en/faculty Armenian National Agrarian University https://anau.am/en/international-relations/ https://anau.am/en/international-relations/trainings-in-foreign-languages/ https://anau.am/en/international-relations/visa-and-insurance/ AZERBAIJAN Baku Higher Oil School (BHOS) http://www.bhos.edu.az/en/page/18-faculty-of-chemical-engineering http://www.bhos.edu.az/en/page/262-faculty-of-petrolium-engineering http://www.bhos.edu.az/en/page/20-faculty-of-proccessing-automation-engineering http://www.bhos.edu.az/nodupload/editor/files/InformationSecurity_Curriculum_Plan.pdf Azerbaijan State University of Economics (UNEC) http://unec.edu.az/en/education/training-programs/ http://unec.edu.az/en/777-international-school-of-economics/ BOSNIA Y HERZEGOVINA University of Sarajevo https://www.unsa.ba/en/org-jedinica http://international.unsa.ba/incoming International Burch University https://www.ibu.edu.ba/faculty-of-engineering-and-natural-sciences/ https://www.ibu.edu.ba/faculty-of-economics-and-social-sciences/ -

International Journal of Humanities and Cultural Studies ISSN 2356-5926
Volume 2 Issue 2 International Journal of Humanities and Cultural Studies ISSN 2356-5926 Editor-in-Chief : Dr. Hassen ZRIBA July-September 2015 Volume 2 Issue 2 Volume 2 Issue 2 INTERNATIONAL JOURNAL OF HUMANITIES AND September 2015 CULTURAL STUDIES ISSN 2356-5926 Editorial Board Editor-in-Chief Managing Editor Dr. Hassen Zriba Najoua Chalbi *Emeritus Professor Ralph Grillo *Dr. Syed Zamanat Abbas University of Sussex, UK Salman Bin Abdulaziz University, Saudi Arabia *Professor Muhammad Asif *Dr. Santosh Kumar Behera Riphah International University, Pakistan Sidho-Kanho-Birsha University, India *Professor Sadok Bouhlila *Dr. P. Prayer Elmo Raj Northern Borders University, Saudi Arabia Pachaiyappa's College, India *Professor Pacha Malyadri *Omid Akhavan Osmania University, Andhra Pradesh, India Imam Ali University, Iran *Professor Shormishtha Panja *Loredana Terec-Vlad University of Delhi, India Ștefan cel Mare University, Romania *Professor Jason L. Powell *Shama Adams University of Chester, UK Curtin University, Australia *Professor Ali H. Raddaoui *Mansour Amini University of Wyoming, USA The Gulf College, Oman *Dr. Mohamed El-Kamel Bakari *Mohd AB Malek Bin MD Shah University of King Abdulaziz, Saudi Arabia Universiti Teknologi Mara, Malaysia *Dr. Solange Barros *Mark B. Ulla Federal University of Mato Grosso, Brazil Father Saturnino Urios University, Philipinnes *Dr. Salah Belhassen * Anouar Bennani University of Gafsa, Tunisia University of Sfax, Tunisia *Dr. Nodhar Hammami Ben Fradj *Shuv Raj Rana Bhat University of Kairouan, Tunisia Central Department of English Kirtipur, Kathmandu, Nepal *Dr. Arbind Kumar Choudhary *Erika Ashley Couto Rangachahi College, Majuli ,Assam, India University of Concordia, Canada *Dr. Amitabh Vikram Dwivedi *Md. Amir Hossain University of Shri Mata Vaishno Devi, India IBAIS University, Bangladesh *Dr. -

UNIMED Member Universities
ASSOCIATED UNIVERSITIES UNIMED represents 138 members from 23 countries ALBANIA . University Paris 1 Panthéon- Sorbonne . European University of Tirana . Metropolitan University of Tirana GREECE . University of Aleksandër Moisiu Durrës . National and Kapodistrian University ALGERIA of Athens . University of Algiers 1 IRAQ . University of Bouira . University of El Oued . Duhok Polytechnic University . University of Mohamed Khider Biskra . Middle Technical University . University of Sétif 2 . University of Duhok . Wasit University CYPRUS ITALY . Cyprus University of Technology . Girne American University . Foro Italico University of Rome . Neapolis University Pafos . Gabriele D'Annunzio University of . University of Cyprus Chieti-Pescara . International Telematic University EGYPT UNINETTUNO . International University of Language . Arab Academy for Science and and Media-IULM Technology and Maritime Transport . International University of Rome- . University of Alexandria UNINT . University of Cairo . IAUV University of Venice . University of Damanhour . Libera Università Mediterranea . University of Sadat City . Link Campus University . Marche Polytechnic University FINLAND . Mercatorum University . Pegaso Telematic University . Tampere University . Polytechnic of Turin . Sapienza University of Rome FRANCE . Tuscia University . University Cà Foscari . Aix-Marseille University . University of Bari . University of Montpellier . University of Bologna . University of Rouen . University of Cagliari . University of Strasbourg . University of Calabria UNIMED - Mediterranean Universities Union Corso Vittorio Emanuele II, 244 |00186 Rome (Italy) | Tel. +39 06 68581430 [email protected] | www.uni-med.net . University of Catania . University of Elmergib . University for Foreigners of Perugia . University of Gharyan . University of Messina . University of Sabratha . University of Modena and Reggio Emilia . University of Sirte . University of Padova . University of Tobruk . University of Palermo . University of Tripoli . University of Pavia . -
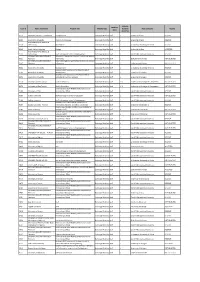
User Id Home Institution Program Title Mobility Type Duration Host University Results Level (Months)
Mobility Mobility User id Home Institution Program title Mobility type Duration Host university Results level (months) 9329 University Hassan II - Casablanca Interpretation Exchange Mobility Staff 1 University of Graz RESERVE 9335 Alexandria University Classical Archaeology Exchange Mobility Staff 1 University of Graz RESERVE Arab Academy for Science & 9336 Technology Matemathics Exchange Mobility Staff 1 La Sapienza University of Rome RESERVE 9346 South Valley Univeristy Biology Exchange Mobility Staff 1 University of Graz ACCEPTED Arab Academy for Science & 9349 Technology Staff Exchange in School of Management Exchange Mobility Staff 1 Cardiff Metropolitan University RESERVE University AbouBekr Belkaid of Automatic Engineering Robotics Control and Applied 9351 Tlemcem Informatics Exchange Mobility Staff 1 Ecole Centrale Nantes NOT SELECTED University AbouBekr Belkaid of Automatic Engineering Robotics Control and Applied 9351 Tlemcem Informatics Exchange Mobility Staff 1 Ecole Centrale Nantes NOT SELECTED 9352 Alexandria University Management Exchange Mobility Staff 1 La Sapienza University of Rome RESERVE Academic Staff position at the Department of 9352 Alexandria University Management Exchange Mobility Staff 1 University of Bologna RESERVE Academic Staff position at the Department of 9372 Alexandria University Agricultural and Food sciences Exchange Mobility Staff 1 University of Bologna RESERVE 9378 University of Sfax Tunisia Staff in Chemistry Exchange Mobility Staff 1-3 University of Santiago de Compostela NOT SELECTED 9378 University -

Promoting Graduate Entrepreneurship in Tunisian Universities
Entrepreneurship, SMEs and Local Development OECD Reviews on Skills and Competences for Entrepreneurship PROMOTING GRADUATE ENTREPRENEURSHIP IN TUNISIAN UNIVERSITIES A REPORT BY THE OECD LOCAL ECONOMIC AND EMPLOYMENT DEVELOPMENT (LEED) PROGRAMME ENTREPRENEURSHIP, SMEs AND LOCAL DEVELOPMENT OECD REVIEWS ON SKILLS AND COMPETENCES FOR ENTREPRENEURSHIP PROMOTING GRADUATE ENTREPRENEURSHIP IN TUNISIAN UNIVERSITIES ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT The OECD is a unique forum where governments work together to address the economic, social and governance challenges of globalisation as well as to exploit its opportunities (www.oecd.org/about). The Organisation provides a setting where governments can compare policy experiences, seek answers to common problems, identify good practice and co-ordinate domestic and international policies. The OECD member countries are: Australia, Austria, Belgium, Canada, Chile, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Japan, Korea, Luxembourg, Mexico, the Netherlands, New Zealand, Norway, Poland, Portugal, the Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, the United Kingdom and the United States. The European Commission takes part in the work of the OECD. This work is published on the responsibility of the Secretary-General of the OECD. The opinions expressed and arguments employed herein do not necessarily reflect the official views of the Organisation or of the governments of its member countries. © OECD 2012 You can copy, download or print OECD content for your own use, and you can include excerpts from OECD publications, databases and multimedia products in your own documents, presentations, blogs, websites and teaching materials, provided that suitable acknowledgment of OECD as source and copyright owner is given. -

Hajer Gueldich
HAJER GUELDICH PERSONAL SITUATION Date of Birth : 4 April 1976 PlaCe of birth : Tunis Nationality : Tunisian Marital status : Married, 3 Children ID : n°07022430 Passport : n° F288953 E-mail address : [email protected] Tweeter: @HajerGueldiCh Phone numbers : (offiCe) : (+216) 71 72 57 36 (Cellular) : (+216) 98 95 18 87 Professional Address : FaCulty of legal, politiCal and soCial scienCes of Tunis 14, Hédi Karray Street, Centre Urbain Nord BP. 40 1080 Tunis, Tunisia Personnel Address : Appartement 021 Immeuble Rayhane 3, Riadh Soukra 2, Ain Zaghouan, 2045, Tunis, Tunisia ( (cellular) : (+216) 98 95 18 87 ( (Office) : (+216) 71 72 57 36 * [email protected] PROFESSIONNAL EXPERIENCE 2018-2017 : Faculty of legal, political and social sciences of Tunis- University of Carthage 2017-2013: Institute of Legal and political sciences of Kairouan- University of Kairouan Professor (PubliC Law) 2 2018-2017: Panafrican University, institute of Governance, Human and social sciences, Yaounde (Cameroon) 2017-2016 : European Inter-University Center for human rights and democratization (EIUC) Venice (Italy) 2015-2014: University of Angers- Faculty of Law (France) 2013-2012: University Loyala- Chicago (USA) Visitor Professor (PubliC international Law) 2018-2016: Institute of political studies- European University of Tunis 2016-2013 : Faculty of legal, political and social sciences of Tunis- University of Carthage 2014-2013 : Faculty of Law and political sciences of Sousse- University of Sousse 2012-2011 : Central University- Private Institute -

Building a Holistic International Educational Partnership: Collaboration Between the University of Georgia and the Tunisian Higher Education System Takoi K
Journal of Community Engagement and Scholarship Volume 4 | Issue 1 Article 2 January 2011 Building a Holistic International Educational Partnership: Collaboration Between The University of Georgia and the Tunisian Higher Education System Takoi K. Hamrita University of Georgia Follow this and additional works at: https://digitalcommons.northgeorgia.edu/jces Recommended Citation Hamrita, Takoi K. (2011) "Building a Holistic International Educational Partnership: Collaboration Between The nivU ersity of Georgia and the Tunisian Higher Education System," Journal of Community Engagement and Scholarship: Vol. 4 : Iss. 1 , Article 2. Available at: https://digitalcommons.northgeorgia.edu/jces/vol4/iss1/2 This Article is brought to you for free and open access by Nighthawks Open Institutional Repository. It has been accepted for inclusion in Journal of Community Engagement and Scholarship by an authorized editor of Nighthawks Open Institutional Repository. Building a HolisticHamrita: International Building a Holistic International Educational Educational Partnership: Partnership: Collab Collaboration Between The University of Georgia and the Tunisian Higher Education System Takoi K. Hamrita Abstract This article reports on a capacity building partnership between The University of Georgia and the higher education system of Tunisia that has been ongoing since 2002. The article discusses important aspects of the program, highlights the conceptual framework and underlying principles that have guided and shaped its design, and gives a comprehensive overview of its overall objectives, concrete actions, and outcomes. Our team’s response to Tunisia’s most urgent development needs; integrating institutional and national resources; building networks of decision makers, administrators, faculty, and students across disciplinary and institutional boundaries; and facilitating the development of indigenous expertise were among the attributes leading to the program’s selection for the Andrew Heiskel Award for Innovation in International Education. -

Membres UNIMED
ASSOCIATED UNIVERSITIES UNIMED represents 141 members from 23 countries ALBANIA . University Paris 1 Panthéon- Sorbonne . European University of Tirana . Metropolitan University of Tirana GREECE . University of Aleksandër Moisiu Durrës . National and Kapodistrian University ALGERIA of Athens . University of Algiers 1 IRAQ . University of Bouira . University of El Oued . Duhok Polytechnic University . University of Mohamed Khider Biskra . Middle Technical University . University of Sétif 2 . University of Duhok CYPRUS ITALY . Cyprus University of Technology . Foro Italico University of Rome . Girne American University . Gabriele D'Annunzio University of . Neapolis University Pafos Chieti-Pescara . University of Cyprus . International Telematic University UNINETTUNO EGYPT . International University of Language and Media-IULM . Arab Academy for Science and . International University of Rome- Technology and Maritime Transport UNINT . University of Alexandria . IAUV University of Venice . University of Cairo . Libera Università Mediterranea . University of Damanhour . Link Campus University . University of Sadat City . Marche Polytechnic University . Mercatorum University FINLAND . Pegaso Telematic University . Polytechnic of Turin . Tampere University . Sapienza University of Rome . Tuscia University FRANCE . University Cà Foscari . University of Bari . Aix-Marseille University . University of Bologna . University of Montpellier . University of Cagliari . University of Rouen . University of Calabria . University of Strasbourg . University of Catania UNIMED - Mediterranean Universities Union Corso Vittorio Emanuele II, 244 |00186 Rome (Italy) | Tel. +39 06 68581430 [email protected] | www.uni-med.net . University for Foreigners of Perugia . Nalut University . University of Messina . Omar Al-Mukhtar University . University of Modena and Reggio Emilia . Sabratha University . University of Padova . Sebha University . University of Palermo . University of Aljufra . University of Pavia . University of Benghazi . University of Perugia . University of Elmergib .