Second!Generation!Antipsychotics!
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

World Journal of Pharmaceutical Research Mathur Et Al
World Journal of Pharmaceutical Research Mathur et al . World Journal of Pharmaceutical SJIF ResearchImpact Factor 8.074 Volume 7, Issue 16, 111-124. Review Article ISSN 2277– 7105 ADRS DUE TO ANTIPSYCHOTIC DRUGS: A REVIEW Prashant Mathur*1 and Waseem Yahya2 1,2Department of Pharmacy Practice Shri Guru Ram Rai Institute of Technology and Science, Patel Nagar (248001), Dehradun, Uttarakhand. INTRODUCTION Article Received on 28 June 2018, WHO defines an ADR as ―any response to a drug which is noxious and Revised on 18 July 2018, unintended, and which occurs at doses normally used in man for Accepted on 08 August 2018, DOI: 10.20959/wjpr201816-12706 prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function‖.[1] The antipsychotic drugs are chemically *Corresponding Author diverse but have the common property of alleviating the symptoms of [2] Prashant Mathur organic as well as functional psychosis. Antipsychotics are among Department of Pharmacy the most effective drugs used in psychiatry in the maintenance therapy Practice Shri Guru Ram Rai of schizophrenia, mania, or in acute psychotic reactions.[3] These drugs Institute of Technology and are also capable of causing a wide range of potential adverse drug Science, Patel Nagar (248001), Dehradun, reactions that can lead to non-compliance that can impair quality of Uttarakhand. life, may cause stigma and physical morbidity which may lead to discontinuation of medication and in extreme cases may be fatal.[4] Antipsychotic drugs have a high therapeutic -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

Maternal Use of Psychiatric Medications During Pregnancy And
MATERNAL USE OF PSYCHIATRIC MEDICATIONS DURING PREGNANCY AND ADVERSE BIRTH OUTCOMES AND NEURODEVELOPMENTAL PROBLEMS IN OFFSPRING Ayesha C. Sujan Submitted to the faculty of the University Graduate School in partial fulfillment of the requirements for the degree Doctor of Philosophy in the Department of Psychological and Brain Sciences, Indiana University July 2021 ii Accepted by the Graduate Faculty, Indiana University, in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Doctoral Committee _______________________________________________ Brian M. D’Onofrio, PhD _______________________________________________ Richard Viken, PhD _______________________________________________ Patrick D. Quinn, PhD _______________________________________________ Christina Ludema, PhD _______________________________________________ A. Sara Oberg, PhD, MD April 22nd, 2020 iii © 2021 Ayesha C. Sujan iv Ayesha Sujan MATERNAL USE OF PSYCHIATRIC MEDICATIONS DURING PREGNANCY AND ADVERSE BIRTH OUTCOMES AND NEURODEVELOPMENTAL PROBLEMS IN OFFSPRING Understanding consequences of prenatal exposure to psychiatric and analgesic medications is important because use of these medications among pregnant women is relatively common and increasing. Rodent experiments have shown effects of perinatal exposure to specific medications; however, these findings might not apply to humans. Human observational studies have been used to study prenatal exposure to psychiatric and analgesic medications rather than randomiZed control trials due to ethical concerns -

When Taking Medication May Be a Sin: Dietary Requirements and Food Laws
BJPsych Advances (2015), vol. 21, 425–432 doi: 10.1192/apt.bp.114.012534 When taking medication may be a ARTICLE sin: dietary requirements and food laws in psychotropic prescribing Waqqas A. Khokhar, Simon L. Dein, Mohammed S. Qureshi, Imran Hameed, Mohammed M. Ali, Yasir Abbasi, Hasan Aman & Ruchit Sood In psychiatric practice, it is not uncommon to Waqqas A. Khokhar is a SUMMARY come across patients who refuse any psychiatric consultant psychiatrist with Leicestershire Partnership NHS Religious laws do not usually forbid the use of intervention, placing their faith in a spiritual psychotropic medication, but many do forbid the Trust and an honorary lecturer in recovery enabled by their religious beliefs (Campion the Centre for Ageing and Mental consumption of animal-based derivatives of bovine 1997; Koenig 2001). Although religious laws do Health at Staffordshire University. and/or porcine origin (e.g. gelatin and stearic not usually restrict the taking of psychotropic Simon L. Dein is a senior lecturer acid) such as are found in many medications. at University College London, an Demonstrating awareness of this, combined with medication, many do forbid consumption of the Honorary Clinical Professor at a genuine concern about how it affects the patient, animal derivatives, particularly gelatin and stearic the University of Durham and a may strengthen the doctor–patient relationship acid, that many psychotropics contain. This has consultant psychiatrist with the and avoid non-adherence. In this article, we major implications, for example, for patients who North Essex Partnership University NHS Foundation Trust. Mohammed outline dietary requirements of key religions and follow Judaism, Islam, Hinduism, Buddhism and S. -

Yo U Are P Ro H Ib Ited from M Aking Th Is P D F P U B Licly Availab Le. It Is
Focus on Women’s Mental Health It is illegal to post this copyrighted PDF on any website. CME Background Depression and Anxiety Articles are selected for credit designation based on an assessment of the educational needs of CME in the Postpartum Period participants, with the purpose of providing readers with a curriculum of CME articles on a variety of and Risk of Bipolar Disorder: topics throughout each volume. Activities are planned using a process that links identified needs A Danish Nationwide with desired results. Register-Based Cohort Study To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Xiaoqin Liu, PhDa,*; Esben Agerbo, DrMedSca,b,c; Evaluation. A $5 processing fee will apply. Jiong Li, PhDd; Samantha Meltzer-Brody, MPHe; Veerle Bergink, PhDa,f,‡; and Trine Munk-Olsen, PhDa,‡ CME Objective After studying this article, you should be able to: • Screen patients with postpartum depressive ABSTRACT symptoms for bipolarity Objective: The first-onset affective episode requiring inpatient treatment in the postpartum period can be a marker of bipolar disorder, but it is Accreditation Statement unknown whether milder postpartum affective episodes are also indicators of underlying bipolarity. Therefore, we aimed to study whether women with a The CME Institute of Physicians nonpsychotic postpartum affective episode treated with antidepressants have Postgraduate Press, Inc., is an increased risk of bipolar disorder. accredited by the Accreditation Council for Continuing Medical Methods: A register-based cohort study was conducted in Denmark of Education to provide continuing 122,622 parous women without psychiatric history who received a first-time medical education for physicians. -

Safety and Effectiveness of Pharmacotherapy for Depression in Adults Who Have Sustained a Traumatic Brain Injury: a Systematic Review Protocol
SYSTEMATIC REVIEW PROTOCOL Safety and effectiveness of pharmacotherapy for depression in adults who have sustained a traumatic brain injury: a systematic review protocol 1,2 1 3 1 2 4 Fiona J. Clay Luke A. Perry Amelia J. Hicks Rachel Batty Catalin Tufanaru Mahesh Jayaram 3,5 6 Jennie Ponsford Malcolm Hopwood 1Department of Psychiatry, Melbourne Neuropsychiatric Centre, The University of Melbourne, Melbourne, Australia, 2The Joanna Briggs Institute, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, Australia, 3Monash-Epworth Rehabilitation Research Centre, Monash Institute of Cognitive and Clinical Neurosciences, Monash University, Melbourne, Australia, 4Department of Psychiatry, University of Melbourne, Royal Melbourne Hospital, Melbourne, Australia, 5School of Psychological Sciences, Monash University, Melbourne, Australia, and 6Professorial Psychiatry Unit, Department of Psychiatry, University of Melbourne, Melbourne, Australia Review objective/question: The objective of this systematic review is to synthesize the current evidence on the effectiveness and harms of pharmacotherapy in the management of depression in adults who have sustained a traumatic brain injury. Keywords Depression; depressive symptomatology; pharmacotherapy; traumatic brain injury JBI Database System Rev Implement Rep 2017; 15(9):2270–2286. Background of TBI survivors report ongoing neurobehavioral and emotional difficulties which may include depres- raumatic brain injury (TBI) comprises a clini- 6 cally heterogeneous group of disorders caused sive symptomatology. Studies indicate that neuro- T behavioral and emotional disturbances impair by trauma inflicted on the brain by a mechanical 7,8 force of external origin. Traumatic brain injury is quality of life the most for TBI survivors. one of the most common causes of death and dis- Depression is one of the most common and dis- abling psychiatric diagnoses for individuals with ability worldwide. -

ORIGINAL ARTICLE Pharmacotherapy of Schizophrenia: the American Current Status Winston W Shen
ORIGINAL ARTICLE Pharmacotherapy of Schizophrenia: The American Current Status Winston W Shen Department of Psychiatry and Human Behavior, Saint Louis University School of Medicine, St Louis, MO, USA (Receivedfor publicationon August22, 1994) Abstract. This is a review paper covering the American current status of pharmacotherapy of schizo phrenia. The author lists all available antipsychotic agents on the market in the United States and describes the American prescribing pattern of antipsychotic agents. This includes a brief history of antipsychotic use in America, acute treatment and chronic maintenance with antipsychotic drugs, the recent advent of atypical antipsychotic agents, and management of antipsychotic-induced side-effects. The characteristics of prescribing American antipsychotics in America are described, and they are then compared with Japanese prescribing practices. The author also makes brief remarks about the uncovered issues in antipsychotic pharmacotherapy and about atypical antipsychotic agents in the context of the future pharmaceutical development. (Keio J Med 43 (4): 192-200, December 1994) Key words: antipsychotics, atypical antipsychotics, psychopharmacology, American prescribing pattern, schizophrenia Introduction Available Antipsychotic Agents on the US Market This paper is a brief review which deals with research Table 1 is a list of antipsychotic agents which are findings, clinical issues and strategies in the pharmaco commonly prescribed in the US. The numbers of potency logical treatments for "Schizophrenia and Other Psy equivalent dose in mg listed in Table 1 are from various chotic Disorders" as one of new 15 DSM-IV Axis I sources and are often inconsistent. Promazine and reser diagnostic categories.1 The diagnoses (and their codes) pine are available in America but are omitted from include schizophrenia (395.xx, 5 types), schizophreniform the list due to their inferior antipsychotic effects. -

Safety Data Sheet - Version 5.0 Preparation Date 10/20/2016 Latest Revision Date (If Revised) SDS Expiry Date 10/19/2019
Safety Data Sheet - Version 5.0 Preparation Date 10/20/2016 Latest Revision Date (If Revised) SDS Expiry Date 10/19/2019 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1 Product Identifier Chemical Name (Z)-Thiothixene Catalogue # T375275 1.2 Relevant Identified Uses of the Substance or Mixture and Uses Advised Against Product Uses To be used only for scientific research and development. Not for use in humans or animals. 1.3 Details of the Supplier of the Safety Data Sheet Company Toronto Research Chemicals 2 Brisbane Road Toronto, ON M3J 2J8 CANADA Telephone +14166659696 FAX +14166654439 Email [email protected] 1.4 Emergency Telephone Number Emergency# +14166659696 between 0800-1700 (GMT-5) 2. HAZARDS IDENTIFICATION WHMIS Classification (Canada) WHMIS Symbols (Canada) None Not WHMIS controlled. 2.1/2.2 Classification of the Substance or Mixture and Label Elements GHS Hazards Classification (According to EU Regulation 1272/2008 and US OSHA 1910.1200) Acute Toxicity, Oral (Category 4) EU Classification (According to EU Regulation 67/548/EEC) Harmful if swallowed. EU Risk and Safety Statements (According to EU Regulation 67/548/EEC) Hazard Statements Hazard Codes Harmful Xn Risk Codes and Phrases R22 Harmful if swallowed. Safety Precaution Codes and Phrases GHS Hazards Identification (According to EU Regulation 1272/2008 and US OSHA 1910.1200) Signal Word Warning GHS Hazard Statements H302 Harmful if swallowed. Toronto Research Chemicals - T375275 Page 1 This Safety Data Sheet contains 16 sections. All 16 sections must be present for this document to be valid. GHS Precautionary Statements P264 Wash hands thoroughly after handling. -
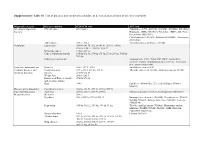
Supplementary Table S1. List of Diseases and Conditions Candidate to Be Tested As Predictors of One-Year Mortality
Supplementary Table S1. List of diseases and conditions candidate to be tested as predictors of one-year mortality Diagnostic category Disease/condition ICD-9 CM code ATC code Infectious and parasitic HIV infection 042.x-044.x Zidovudine (AZT) (J05AF01, J05AR01, J05AR04, J05AR05), diseases Didanosine (DDI) (J05AF02), Zalcitabine (DDC) (J05AF03), Pentamidine (P01CX01), Clarithromycin (J01FA09), Rifabutin (J04AB04), Atovaquone (P01AX06) Tuberculosis 010.x - 018.x Anti-tuberculosis antibiotics (J04AB) Neoplasms Lymphoma 200.00-202.38, 202.50-203.01, 203.8x, 238.6x, 273.3x, V10.71, V10.72, V10.79 Metastatic cancer 196.0x-199.1x Cancer, without metastasis 140.0x-172.9x, 174.0x-175.9x,179.x-195.8x, V10.0x- V10.9x Malignancy medication Antineoplastic (L01), Taxol (C07AB05), Interleukins (L03AC), Colony-stimulating factors (L03AA), Antinausea misc, ondansetron (A04) Endocrine, nutritional and Diabetes 250.x, 357.2, 362.0 Antidiabetic agents (A10) metabolic diseases, and Hypothyroidism 243.x-244.2, 244.8x, 244.9x Thyroid replacement (H03A), Antithyroid agents (H03B) immunity disorders Obesity 278.00-278.01 Weight loss 260.0x-263.9 Disorders of fluid, electrolyte, 276.0x-276.9x and acid-base balance Gout 274.x Colchicine (M04AC01), Uric acid inhibitors (M04AA, M04AB) Diseases of the blood and Coagulation defects 286.0x-286.9x, 287.1x, 287.3x-287.5x blood-forming organs Anaemias 280.0x, 280.1x-281.9x, 285.9x Marrow stimulants (L03AA), Erythropoietin (B03XA01) Mental disorders Dementia 290.x Psychosis 295.x-298.9x, 299.10-299.11 Butyrophenone derivates -

Antipsychotics-Children-Surveillance
AHRQ Comparative Effectiveness Review Surveillance Program CER #39: First & second generation antipsychotics for children and young adults Original Release Date: February, 2012 Surveillance Report: November, 2012 Surveillance Report: October, 2014 Summary of Key Findings from Surveillance Reports: • For Key Question 1, studies were identified suggesting that conclusions related to the effectiveness of Second Generation Antidepressants (SGA) for anorexia nervosa are out of date, that conclusions related to the comparative effectiveness of First Generation Antidepressants (FGA) and SGAs are possibly out of date, that the conclusion of no difference between SGAs and placebo for aggression is possibly out of date, that the strength of evidence (SOE) for SGAs and improvement on mania scales may possibly increase from low to moderate, and the SOE regarding risperidone and reduction in problem behavior may have increased. • For Key Question 2, conclusions related to cardiovascular events and weight gain, and the tolerability of molidone hydrochloride are possibly out of date, and the SOE for additional adverse events may possibly increase from moderate to high. • For Key Question 3, the conclusion on the safety and tolerability of FGAs vs. SGAs is possibly out of date. • For Key Question 4, conclusions related to individuals with comorbid disorders are possibly out of date. Signal Assessment: The signals examined in this surveillance assessment suggest that the original CER is possibly out of date. i Authors: Karli Kondo Maya O’Neil Shammarie Mathis Ryan McKenna Kelly Vander Ley Johanna Anderson Mark Helfand Conflict of Interest: None of the investigators has any affiliations or financial involvement that conflicts with the material presented in this report. -

Electronic Search Strategies
Appendix 1: electronic search strategies The following search strategy will be applied in PubMed: ("Antipsychotic Agents"[Mesh] OR acepromazine OR acetophenazine OR amisulpride OR aripiprazole OR asenapine OR benperidol OR bromperidol OR butaperazine OR Chlorpromazine OR chlorproethazine OR chlorprothixene OR clopenthixol OR clotiapine OR clozapine OR cyamemazine OR dixyrazine OR droperidol OR fluanisone OR flupentixol OR fluphenazine OR fluspirilene OR haloperidol OR iloperidone OR levomepromazine OR levosulpiride OR loxapine OR lurasidone OR melperone OR mesoridazine OR molindone OR moperone OR mosapramine OR olanzapine OR oxypertine OR paliperidone OR penfluridol OR perazine OR periciazine OR perphenazine OR pimozide OR pipamperone OR pipotiazine OR prochlorperazine OR promazine OR prothipendyl OR quetiapine OR remoxipride OR risperidone OR sertindole OR sulpiride OR sultopride OR tiapride OR thiopropazate OR thioproperazine OR thioridazine OR tiotixene OR trifluoperazine OR trifluperidol OR triflupromazine OR veralipride OR ziprasidone OR zotepine OR zuclopenthixol ) AND ("Randomized Controlled Trial"[ptyp] OR "Controlled Clinical Trial"[ptyp] OR "Multicenter Study"[ptyp] OR "randomized"[tiab] OR "randomised"[tiab] OR "placebo"[tiab] OR "randomly"[tiab] OR "trial"[tiab] OR controlled[ti] OR randomized controlled trials[mh] OR random allocation[mh] OR double-blind method[mh] OR single-blind method[mh] OR "Clinical Trial"[Ptyp] OR "Clinical Trials as Topic"[Mesh]) AND (((Schizo*[tiab] OR Psychosis[tiab] OR psychoses[tiab] OR psychotic[tiab] OR disturbed[tiab] OR paranoid[tiab] OR paranoia[tiab]) AND ((Child*[ti] OR Paediatric[ti] OR Pediatric[ti] OR Juvenile[ti] OR Youth[ti] OR Young[ti] OR Adolesc*[ti] OR Teenage*[ti] OR early*[ti] OR kids[ti] OR infant*[ti] OR toddler*[ti] OR boys[ti] OR girls[ti] OR "Child"[Mesh]) OR "Infant"[Mesh])) OR ("Schizophrenia, Childhood"[Mesh])).