I Element Periodic Table
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Package 'Ciaawconsensus'
Package ‘CIAAWconsensus’ September 19, 2018 Type Package Title Isotope Ratio Meta-Analysis Version 1.3 Author Juris Meija and Antonio Possolo Maintainer Juris Meija <[email protected]> Description Calculation of consensus values for atomic weights, isotope amount ratios, and iso- topic abundances with the associated uncertainties using multivariate meta-regression ap- proach for consensus building. License Unlimited LazyData yes Imports mvtnorm, stringr, numDeriv, stats, Matrix NeedsCompilation no Repository CRAN Date/Publication 2018-09-19 13:30:12 UTC R topics documented: abundances2ratios . .2 at.weight . .3 ciaaw.mass.2003 . .4 ciaaw.mass.2012 . .5 ciaaw.mass.2016 . .6 iridium.data . .6 mmm ............................................7 normalize.ratios . .8 platinum.data . .9 Index 10 1 2 abundances2ratios abundances2ratios Isotope ratios of a chemical element from isotopic abundances Description This function calculates the isotope ratios of a chemical element from the given isotopic abundances and their uncertainties. The uncertainty evaluation is done using the propagation of uncertainty and the missing correlations between the isotopic abundances are reconstructed using Monte Carlo methods. Usage abundances2ratios(x, ux, ref=1, iterations=1e4) Arguments x A vector of isotopic abundances of an element ux Standard uncertainties of x ref Index to specify the desired reference isotope for isotope amount ratios iterations Number of iterations for isotopic abundance correlation mapping Details Situations are often encountered where isotopic abundances are reported but not the isotope ratios. In such cases we reconstruct the isotope ratios that are consistent with the abundances and their uncertainties. Given only the abundances and their uncertainties, for elements with four or more isotopes one cannot unambiguously infer the uncertainties of the ratios due to the unknown correla- tions between isotopic abundances. -

CHEMISTRY for the Bottom of the Periodic Table
http://cyclotron.tamu.edu CHEMISTRY for the Bottom of the Periodic Table Techniques to investigate chemical properties of superheavy elements lead to improved methods for separating heavy metals THE SCIENCE The chemical properties of superheavy element 113, nihonium, are almost completely unknown, so a team of researchers from the Cyclotron Institute at Texas A&M University and the Institut Pluridisciplinaire Hubert Curien in France are developing techniques that could be used to study this fleeting element. As part of that effort, they are comparing the properties of nihonium to the chemically similar elements indium and thallium; to do so, the team studied the separation of these two elements using a new class of designer molecules called ionic liquids. THE IMPACT Measuring the chemical properties of nihonium and other superheavy elements will increase our understanding of the principles that control the Periodic Table. Comparing the data from nihonium to results for similar elements, obtained using the team’s fast, efficient, single-step process, reveals trends that arise from the structure of the Periodic Table. This research could also lead to better methods of re- using indium, a metal that is part of flat-panel displays but not currently mined in the United States. The proposed mechanism of transfer. Thallium (Tl) bonds with chlorine (Cl) and moves into the ionic liquid (in blue). SUMMARY The distribution of indium and thallium between the aque- ous and organic phases is the key to understanding the PUBLICATIONS separation of these elements. An aqueous solution, con- E.E. Tereshatov, M. Yu. Boltoeva, V. Mazan, M.F. -

Thermodynamics of Ion Exchange
Chapter 1 Thermodynamics of Ion Exchange Ayben Kilislioğlu Additional information is available at the end of the chapter http://dx.doi.org/10.5772/53558 1. Introduction 1.1. Ion exchange equilibria During an ion exchange process, ions are essentially stepped from the solvent phase to the solid surface. As the binding of an ion takes place at the solid surface, the rotational and translational freedom of the solute are reduced. Therefore, the entropy change (ΔS) during ion exchange is negative. For ion exchange to be convenient, Gibbs free energy change (ΔG) must be negative, which in turn requires the enthalpy change to be negative because ΔG = ΔH - TΔS. Both enthalpic (ΔHo) and entropic (ΔSo) changes help decide the overall selectivity of the ion-exchange process [Marcus Y., SenGupta A. K. 2004]. Thermodynamics have great efficiency on the impulsion of ion exchange. It also sets the equilibrium distribution of ions between the solution and the solid. A discussion about the role of thermodynamics relevant to both of these phenomena was done by researchers [Araujo R., 2004]. As the basic rule of ion exchange, one type of a free mobile ion of a solution become fixed on the solid surface by releasing a different kind of an ion from the solid surface. It is a reversible process which means that there is no permanent change on the solid surface by the process. Ion exchange has many applications in different fields like enviromental, medical, technological,.. etc. To evaluate the properties and efficiency of the ion exchange one must determine the equilibrium conditions. -

Properties of Carbon the Atomic Element Carbon Has Very Diverse
Properties of Carbon The atomic element carbon has very diverse physical and chemical properties due to the nature of its bonding and atomic arrangement. fig. 1 Allotropes of Carbon Some allotropes of carbon: (a) diamond, (b) graphite, (c) lonsdaleite, (d–f) fullerenes (C60, C540, C70), (g) amorphous carbon, and (h) carbon nanotube. Carbon has several allotropes, or different forms in which it can exist. These allotropes include graphite and diamond, whose properties span a range of extremes. Despite carbon's ability to make 4 bonds and its presence in many compounds, it is highly unreactive under normal conditions. Carbon exists in 2 main isotopes: 12C and 13C. There are many other known isotopes, but they tend to be short-lived and have extremely short half-lives. Allotropes The different forms of a chemical element. Cabon is the chemical element with the symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon has 6 protons and 6 Source URL: https://www.boundless.com/chemistry/nonmetallic-elements/carbon/properties-carbon/ Saylor URL: http://www.saylor.org/courses/chem102#6.1 Attributed to: Boundless www.saylor.org Page 1 of 2 neutrons, and has a standard atomic weight of 12.0107 amu. Its electron configuration is denoted as 1s22s22p2. It is a solid, and sublimes at 3,642 °C. It's oxidation state ranges from 4 to -4, and it has an electronegativity rating of 2.55 on the Pauling scale. Carbon has several allotropes, or different forms in which it exists. -

IUPAC Wire See Also
News and information on IUPAC, its fellows, and member organizations. IUPAC Wire See also www.iupac.org Flerovium and Livermorium Join the Future Earth: Research for Global Periodic Table Sustainability n 30 May 2012, IUPAC officially approved he International Council for Science (ICSU), of the name flerovium, with symbol Fl, for the which IUPAC is a member, announced a new Oelement of atomic number 114 and the name T10-year initiative named Future Earth to unify livermorium, with symbol Lv, for the element of atomic and scale up ICSU-sponsored global environmental- number 116. The names and symbols were proposed change research. by the collaborating team of the Joint Institute for Nuclear Research (Dubna, Russia) and the Lawrence Operational in 2013, this new ICSU initiative will Livermore National Laboratory (Livermore, California, provide a cutting-edge platform to coordinate scien- USA) to whom the priority for the discovery of these tific research to respond to the most critical social and elements was assigned last year. The IUPAC recom- environmental challenges of the 21st century at global mendations presenting these names is to appear in and regional levels. “This initiative will link global envi- the July 2012 issue of Pure and Applied Chemistry. ronmental change and fundamental human develop- ment questions,” said Diana Liverman, co-director of The name flerovium, with symbol Fl, lies within the Institute of the Environment at the University of tradition and honors the Flerov Laboratory of Nuclear Arizona and co-chair of the team Reactions in Dubna, Russia, where the element of that is designing Future Earth. -

Energy and the Hydrogen Economy
Energy and the Hydrogen Economy Ulf Bossel Fuel Cell Consultant Morgenacherstrasse 2F CH-5452 Oberrohrdorf / Switzerland +41-56-496-7292 and Baldur Eliasson ABB Switzerland Ltd. Corporate Research CH-5405 Baden-Dättwil / Switzerland Abstract Between production and use any commercial product is subject to the following processes: packaging, transportation, storage and transfer. The same is true for hydrogen in a “Hydrogen Economy”. Hydrogen has to be packaged by compression or liquefaction, it has to be transported by surface vehicles or pipelines, it has to be stored and transferred. Generated by electrolysis or chemistry, the fuel gas has to go through theses market procedures before it can be used by the customer, even if it is produced locally at filling stations. As there are no environmental or energetic advantages in producing hydrogen from natural gas or other hydrocarbons, we do not consider this option, although hydrogen can be chemically synthesized at relative low cost. In the past, hydrogen production and hydrogen use have been addressed by many, assuming that hydrogen gas is just another gaseous energy carrier and that it can be handled much like natural gas in today’s energy economy. With this study we present an analysis of the energy required to operate a pure hydrogen economy. High-grade electricity from renewable or nuclear sources is needed not only to generate hydrogen, but also for all other essential steps of a hydrogen economy. But because of the molecular structure of hydrogen, a hydrogen infrastructure is much more energy-intensive than a natural gas economy. In this study, the energy consumed by each stage is related to the energy content (higher heating value HHV) of the delivered hydrogen itself. -

The Histories Hidden in the Periodic Table
The Histories Hidden in the Periodic Table From poisoned monks and nuclear bombs to the “transfermium wars,” mapping the atomic world hasn’t been easy. By Neima Jahromi 6:00 A.M. As element hunters have become element makers, the periodic table’s meaning has changed. It now describes what is possible, in addition to what merely exists. Illustration by Ilya Milstein The story of the fifteenth element began in Hamburg, in 1669. The unsuccessful glassblower and alchemist Hennig Brandt was trying to find the philosopher’s stone, a mythical substance that could turn base metals into gold. Instead, he distilled something new. It was foamy and, depending on the preparation, yellow or black. He called it “cold fire,” because it glowed in the dark. Interested parties took a look; some felt that they were in the presence of a miracle. “If anyone had rubbed himself all over with it,” one observer noted, “his whole figure would have shone, as once did that of Moses when he came down from Mt. Sinai.” Robert Boyle, the father of modern chemistry, put some on his hand and noted how “mild and innocent” it seemed. Another scientist saw particles in it twinkling “like little stars.” At first, no one could figure out what the Prometheus of Hamburg had stolen. After one of Brandt’s confidants provided a hint—the main ingredient was “somewhat that belong’d to the Body of Man”—Boyle deduced that he and his peers had been smearing themselves with processed urine. As the Cambridge chemist Peter Wothers explains in his new history of the elements, “Antimony, Gold, and Jupiter’s Wolf” (Oxford), Brandt’s recipe called for a ton of urine. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -

Science-8 Module-8 Version-3.Pdf
Republic of the Philippines Department of Education Regional Office IX, Zamboanga Peninsula 8 SCIENCE Quarter 3 - Module 8 PERIODIC PROPERTIES OF ELEMENTS Name of Learner: ___________________________ Grade & Section: ___________________________ Name of School: ___________________________ Science- Grade 8 Support Material for Independent Learning Engagement (SMILE) Quarter 3 - Module 8: Periodic Properties of Elements First Edition, 2021 Republic Act 8293, section 176 states that: No copyright shall subsist in any work of the Government of the Philippines. However, prior approval of the government agency or office wherein the work is created shall be necessary for the exploitation of such work for a profit. Such agency or office may, among other things, impose as a condition the payment of royalty. Borrowed materials (i.e., songs, stories, poems, pictures, photos, brand names, trademarks, etc.) included in this book are owned by their respective copyright holders. Every effort has been exerted to locate and seek permission to use these materials from their respective copyright owners. The publisher and authors do not represent nor claim ownership over them. Development Team of the Module Writer: Galo M. Salinas Editor: Teodelen S. Aleta Reviewers: Teodelen S. Aleta, Zyhrine P. Mayormita Lay-out Artists: Zyhrine P. Mayormita, Chris Raymund M. Bermudo Management Team: Virgilio P. Batan Jr. - Schools Division Superintendent Lourma I. Poculan - Asst. Schools Division Superintendent Amelinda D. Montero - Chief Education Supervisor, CID Nur N. Hussien - Chief Education Supervisor, SGOD Ronillo S. Yarag - Education Program Supervisor, LRMS Zyhrine P. Mayormita - Education Program Supervisor, Science Leo Martinno O. Alejo - Project Development Officer II, LRMS Janette A. Zamoras - Public Schools District Supervisor Adrian G. -
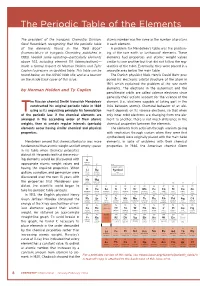
The Periodic Table of the Elements
The Periodic Table of the Elements The president of the Inorganic Chemistry Division, atomic number was the same as the number of protons Gerd Rosenblatt, recognizing that the periodic table in each element. of the elements found in the “Red Book” A problem for Mendeleev’s table was the position- (Nomenclature of Inorganic Chemistry, published in ing of the rare earth or lanthanoid* elements. These 1985) needed some updating—particularly elements elements had properties and atomic weight values above 103, including element 110 (darmstadtium)— similar to one another but that did not follow the reg- made a formal request to Norman Holden and Tyler ularities of the table. Eventually, they were placed in a Coplen to prepare an updated table. This table can be separate area below the main table. found below, on the IUPAC Web site, and as a tear-off The Danish physicist Niels Henrik David Bohr pro- on the inside back cover of this issue. posed his electronic orbital structure of the atom in 1921, which explained the problem of the rare earth by Norman Holden and Ty Coplen elements. The electrons in the outermost and the penultimate orbits are called valence electrons since generally their actions account for the valence of the he Russian chemist Dmitri Ivanovich Mendeleev element (i.e., electrons capable of taking part in the constructed his original periodic table in 1869 links between atoms). Chemical behavior of an ele- Tusing as its organizing principle his formulation ment depends on its valence electrons, so that when of the periodic law: if the chemical elements are only inner orbit electrons are changing from one ele- arranged in the ascending order of their atomic ment to another, there is not much difference in the weights, then at certain regular intervals (periods) chemical properties between the elements. -

Guidelines for the Use of Atomic Weights 5 10 11 12 DOI: ..., Received ...; Accepted
IUPAC Guidelines for the us e of atomic weights For Peer Review Only Journal: Pure and Applied Chemistry Manuscript ID PAC-REC-16-04-01 Manuscript Type: Recommendation Date Submitted by the Author: 01-Apr-2016 Complete List of Authors: van der Veen, Adriaan; VSL Meija, Juris Possolo, Antonio; National Institute of Standards and Technology Hibbert, David; University of New South Wales, School of Chemistry atomic weights, atomic-weight intervals, molecular weight, standard Keywords: atomic weight, measurement uncertainty, uncertainty propagation Author-Supplied Keywords: P.O. 13757, Research Triangle Park, NC (919) 485-8700 Page 1 of 13 IUPAC Pure Appl. Chem. 2016; aop 1 2 3 4 Sponsoring body: IUPAC Inorganic Chemistry Division Committee: see more details on page XXX. 5 IUPAC Recommendation 6 7 Adriaan M. H. van der Veen*, Juris Meija, Antonio Possolo, and D. Brynn Hibbert 8 9 Guidelines for the use of atomic weights 5 10 11 12 DOI: ..., Received ...; accepted ... 13 14 Abstract: Standard atomicFor weights Peer are widely used Review in science, yet the uncertainties Only associated with these 15 values are not well-understood. This recommendation provides guidance on the use of standard atomic 16 weights and their uncertainties. Furthermore, methods are provided for calculating standard uncertainties 17 of molecular weights of substances. Methods are also outlined to compute material-specific atomic weights 10 18 whose associated uncertainty may be smaller than the uncertainty associated with the standard atomic 19 weights. 20 21 Keywords: atomic weights; atomic-weight intervals; molecular weight; standard atomic weight; uncertainty; 22 uncertainty propagation 23 24 25 1 Introduction 15 26 27 Atomic weights provide a practical link the SI base units kilogram and mole. -

(I) Determination of the Equivalent Weight and Pka of an Organic Acid
Experiment 4 (i) Determination of the Equivalent Weight and pKa of an Organic Acid Discussion This experiment is an example of a common research procedure. Chemists often use two or more analytical techniques to study the same system. These experiments can give complementary qualitative and quantitative information concerning an unknown substance. I. Titration of Acids and Bases in Aqueous Solutions The almost instantaneous reaction between acids and bases in aqueous solution produce changes in pH which one can monitor. Two techniques are useful for detecting the equivalence point: (1) colorimetry, using an acid-base color indicator - a dye which undergoes a sharp change in color in a region of pH covering the equivalence point and (2) potentiometry, using a potentiometer (pH meter) to record the sharp change at the equivalence point in the potential difference between an electrode (usually a glass electrode) and the solution whose pH is undergoing change as a result of the addition of acid or base. For example, in the case of the titration of a weak monoprotic acid HA using sodium hydroxide solution we may write: + - NaOH + HA → Na + A + H2O (4.1) Applying the law of mass action to the ionization equilibrium for the weak acid in water: + - HA + H2O H3O + A (4.2) we may write (in dilute solutions [H2O] is essentially constant) [H O+ ][A − ] 3 = K (4.3) [HA] a where Ka is the acid ionization constant (constant at any given temperature). This expression is valid for + - all aqueous solutions containing hydronium ions (H3O ), A ions, and the un-ionized molecules HA.