Abstract Book
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
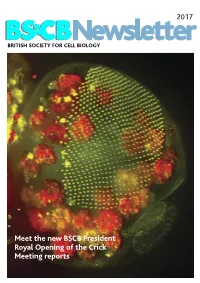
BSCB Newsletter 2017D
2017 BSCB Newsletter BRITISH SOCIETY FOR CELL BIOLOGY Meet the new BSCB President Royal Opening of the Crick Meeting reports 2017 CONTENTS BSCB Newsletter News 2 Book reviews 7 Features 8 Meeting Reports 24 Summer students 30 Society Business 33 Editorial Welcome to the 2017 BSCB newsletter. After several meeting hosted several well received events for our Front cover: years of excellent service, Kate Nobes has stepped PhD and Postdoc members, which we discuss on The head of a Drosophila pupa. The developing down and handed the reins over to me. I’ve enjoyed page 5. Our PhD and Postdoc reps are working hard compound eye (green) is putting together this years’ newsletter. It’s been great to make the event bigger and better for next year! The composed of several hundred simple units called ommatidia to hear what our members have been up to, and I social events were well attended including the now arranged in an extremely hope you will enjoy reading it. infamous annual “Pub Quiz” and disco after the regular array. The giant conference dinner. Members will be relieved to know polyploidy cells of the fat body (red), the fly equivalent of the The 2016 BSCB/DB spring meeting, organised by our we aren’t including any photos from that here. mammalian liver and adipose committee members Buzz Baum (UCL), Silke tissue, occupy a big area of the Robatzek and Steve Royle, had a particular focus on In this issue, we highlight the great work the BSCB head. Cells and Tissue Architecture, Growth & Cell Division, has been doing to engage young scientists. -

Curriculum Vitae Prof. Dr. Geoffrey L. Smith
Curriculum Vitae Prof. Dr. Geoffrey L. Smith Name: Geoffrey L. Smith Born: 23 July 1955 Main areas of research: Microbiology, virology, immunology, vaccinia virus, vaccines Geoffrey L. Smith is a British microbiologist and virologist specialising in the area of poxviruses. His research examines the interaction of poxviruses with the infected host cell and the immune system, concentrating especially on the vaccinia virus that was the vaccine used to eradicate smallpox. His research has enabled new vaccination concepts to be developed and provided important insights into how viruses evade the host innate immune response and cause disease. Academic and Professional Career since 2011 Chair of the Pathology Department, University of Cambridge, UK and Principal Research Fellow, Wellcome Trust 2000 ‐ 2011 Director, Department of Virology, Imperial College London, UK 1989 ‐ 2000 Reader then Professor, University of Oxford, UK 1985 ‐ 1989 Lecturer in Virology, University of Cambridge, UK 1981 ‐ 1984 Postdoctoral Fellow, National Institutes of Health, Bethesda, USA 1981 PhD, National Institute for Medical Research, Mill Hill, London, UK Functions in Scientific Societies and Committees since 2015 Chair of the scientific council, Friedrich Löffler Institute 2012 ‐ 2016 Member, Biomedical Panel of the University Research Grant Committee, Hong Kong 2011 ‐ 2014 President, International Union of Microbiological Societies 2009 ‐ 2012 Chair, Royal Society Committee for Scientific Aspects of International Security Nationale Akademie der Wissenschaften Leopoldina -

Breakthrough How Ideas Exchanged in the Canteen Can Lead to Research Discoveries
News from the Medical Research Council network Summer 2013 100 years of life-changing discoveries The greatcoffee breakthrough How ideas exchanged in the canteen can lead to research discoveries. Discoveries of the century Well-known figures give us their thoughts on the biggest medical advances, past and future. Network can also be downloaded as a PDF at: www.mrc.ac.uk/network CONTENTS NEWS News COMMENT FROM A phenomenal legacy begins 3 A phenomenal John Informatics research centres launched across the UK 3 legacy begins Speedier access to brain tissue for research 5 Savill Universities and Science Minister David Willetts has officially opened the MRC-NIHR Phenome Centre CHIEF EXECUTIVE at Imperial College London, which will re-purpose some of the sophisticated technology from the Royal opening for new LMB building 5 The launch of four e-health London 2012 Olympics Anti-Doping Centre. informatics research centres (eHIRCs) and the UK informatics Unlike our genome, which collectively describes a person’s genetic material, the phenome describes all the other chemistry of our research network by the Science body. It is the product of how our genes and environment interact throughout development and life, and is analysed by linking our Minister in May (see opposite), chemical, metabolic and physiological features and responses. By measuring phenome patterns throughout life, researchers at the Latest discoveries marked part of a £90m total centre aim to identify the patterns which separate those who develop particular diseases from those who don’t. investment in so-called big data or Gout drug offers hope for heart disease patients 10 medical bioinformatics research– something we excel at here in The centre’s first three pilot studies are now underway. -

Sir Bernard Katz (1911-2003): an Icon of Neurophysiology
Curr Neurobiol 2018; 9(3): 101-105 ISSN 0975-9042 Sir Bernard Katz (1911-2003): An Icon of Neurophysiology Ronald P Rubin, PhD Department of Pharmacology & Toxicology, Jacobs School of Medicine & Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, New York Abstract This article highlights the life and career of Sir Bernard Katz, a member of a generation of eminent physiologists who was a refugee from the Third Reich. Despite setbacks incurred by anti- Semitism early in life, Katz had the tenacity to achieve fulfillment in his extraordinary career. With the support of the eminent A.V. Hill, who had a pivotal influence on his life and career, Katz conducted research at University College London mainly in the 1950’s and 60’s. His work included the study of miniature end-plate potentials, quantal secretion of neurotransmitters, the role of calcium in transmitter release, and the postsynaptic action of acetylcholine. In addition, his department was a center for pre- and postdoctoral students from all over the world and his influence on the training of a large number of the world’s most prominent neurophysiologist was monumental. Because Bernard Katz’s work remains the basis of our understanding of the release and actions of neurotransmitters, he was awarded the Nobel Prize in 1970. Keywords: Bernard Katz, Neuromuscular Junction, Quantal release, End-Plate potential. Early Years After the Russian revolution of 1917, the members of the Katz family- along with other Russian expatriates- lost their Sir Bernard Katz was one of the generation of distinguished nationality and became stateless. -
13Th January 1927, Germiston, South Africa Nationality: British Married
SYDNEY BRENNER Born: 13th January 1927, Germiston, South Africa Nationality: British Married, three children (and one step-son) MSc (1947) University of Witwatersrand, South Africa MB, BCh (1951) University of Witwatersrand, South Africa DPhil (1954) Oxford University, England Companion of Honour (1987) National Day Public Service Star, Republic of Singapore (2000) Fellow of King's College, Cambridge (1959- ) Member of Medical Research Council (1978-82; 1986-90) Fellow of the Royal Society (FRS) (1965) Fellow of the Royal College of Physicians (FRCP) (1979) Foreign Honorary Member, American Academy of Arts and Sciences (1965) Fellow of the American Association for the Advancement of Science (1966) Honorary DSc, Trinity College, Dublin (1967) Honorary DSc, University of Witwatersrand (1972) Honorary Member of the Deutsche Akademie der Natursforscher Leopoldina,Germany (1975) Honorary Member of the Society for Biological Chemists (1975) Honorary DSc, University of Chicago (1976) Foreign Associate of the US National Academy of Sciences (1977) Honorary Fellow of the Royal Society of Edinburgh (1979) Foreign Member of the American Philosophical Society (1979) Honorary LLD, University of Glasgow (1981) Honorary DSc, University of London (1982) Honorary DSc, University of Leicester (1983) Foreign Associate, Royal Society of South Africa (1983) Honorary DSc, University of Oxford (1985) Honorary Fellow of Exeter College, Oxford (1985) Foreign Member of Real Academia de Ciencias (Spain) (1985) External Scientific Member of the Max-Planck Society -

International Workshop Assessing the Security Implications of Genome Editing Technology
International Workshop Assessing the Security Implications of Genome Editing Technology 11-13 October, 2017 Herrenhausen Palace ● Hanover, Germany The workshop organizing committee is comprised of 10 international scholars with expertise on genome editing, security implications of biotechnology, science and technology policy, and public engagement in science. The organizing committee is assisted by 6 expert staff from the partnering global academies of sciences. ORGANIZING COMMITTEE Volker ter Meulen, MD (Chair), studied medicine in Münster, Innsbruck (Austria), Kiel and Göttingen, received a training in virology at the University of Philadelphia, USA, and in pediatrics at the University Hospital of Göttingen. In 1975 he became Chairman at the Institute of Virology and Immunobiology, University of Würzburg. His major research investigations have been directed towards the study of the etiology and pathogenesis of persistent virus infections with particular emphasis of the central nervous system. He was twice Dean of the Faculty of Medicine at the University of Würzburg and due to the recognition of his research achievements and his experiences to head a Medical Faculty successfully, Dr. ter Meulen has served as a member of many national and international committees giving scientific advice to policy makers and society. From 2003 – 2010, Dr. ter Meulen was President of the German Academy of Sciences Leopoldina. From 2007 – 2010, he served as president of the European Academies of Science Advisory Council (EASAC). Since 2013, Dr ter Meulen is Co-Chair of IAP: the InterAcademy Partnership, the Global Network of Science and Medical Academies. Baerbel Friedrich, PhD, is the Scientific Director of the Alfried Krupp Institute of Advanced Studies in Greifswald, Germany. -

Professor Bert Sakmann Bert Sakmann Studied at the Universities
Professor Bert Sakmann Bert Sakmann studied at the universities of Tübingen and Munich, graduating in 1967. Much of his professional life has been spent in various branches of the Max-Planck-Institut. A British Council Fellowship took him in 1971 to the Department of Biophysics of University College London to work with Bernard Katz, co-recipient in 1970 of the Nobel Prize in Medicine for discoveries concerning the humoral transmitters in the nerve terminals and the mechanism for their storage, release and inactivation. Bert Sakmann’s admiration for his supervisor was to find tangible expression in 1993, through the establishment of the Bernard Katz Minerva Center for Cell Biophysics, a joint venture of the Max Planck Institute for Medical Research in Heidelberg where Bert Sakmann is Director, the Hebrew University of Jerusalem and the Technicon-Israel Institute of Technology in Haifa. He also established the annual Bernard Katz prize lecture. In 1974, he obtained his PhD from the University of Göttingen and with Erwin Neher, at the Max-Planck-Institut für Biophysikalische Chemie, began the work which was to revolutionise cellular biology and neuroscience and win them the 1991 Nobel Prize for Physiology and Medicine. The patch clamp technique involves attaching tiny pipettes directly to a cell, making possible very precise measurements of the electrical flow. This allows researchers to measure the electrical current going in and out of the ion channels of a cell. His many significant discoveries have revolutionised our knowledge of the workings of cells, particularly nerve cells. Professor Sakmann's important discovery of the patch-clamp technique for measuring electrical activity and chemical flow across cell membranes resulted in a technique now used in laboratories throughout the world. -
Patrick Cramer Curriculum Vitae
Patrick Cramer - Curriculum vitae page 1 Patrick Cramer Curriculum vitae Personal data Date of birth February 3, 1969 Place of birth Stuttgart, Germany Nationality German Marital status Married, two children: Lea Sophie (*1997), Dominik Robin (*2000) Contact information Address Max Planck Institute for Biophysical Chemistry Department of Molecular Biology Am Fassberg 11, 37077 Göttingen, Germany Email [email protected] Phone +49 551 201-2800 Internet www.mpibpc.mpg.de/cramer Twitter @CramerLab Positions 2014- MPI for Biophysical Chemistry Director, Department Molecular Biology 2010-2013 Universität München (LMU) Director, Department of Biochemistry 2004-2013 Universität München (LMU) Director, Gene Center Munich 2004-2014 Universität München (LMU) Professor of Biochemistry 2001-2003 Universität München (LMU) Tenure-track Professor of Biochemistry 1999-2001 Stanford University, USA Postdoctoral fellow with R.D. Kornberg 1995-1998 EMBL Grenoble, France Predoctoral fellow with C.W. Müller Education 1998 EMBL & Universität Heidelberg Dr. rer. nat. (‘summa cum laude’) 1995 Universität Heidelberg Diploma in Chemistry 7/94-3/95 Cambridge University, UK Research student with A.R. Fersht 10/92-3/93 University of Bristol, UK ERASMUS research student with A.G. Orpen 1992-1995 Universität Heidelberg Student of Chemistry (Diplom) 1989-1991 Universität Stuttgart Student of Chemistry (Vordiplom) Publications and public outreach >220 Medline-listed publications, incl. 42 original papers in Nature, Science & Cell >500 invited lectures and seminars, -

MRC Network June/July 2009
News from the Medical Research Council June / July 2009 MRC scientists join front line in global fight against flu outbreak page 2 Profile: The Centre for Cognitive Ageing and Cognitive Epidemiology, Edinburgh page 10 3 JUNE/JULY 2009 of different vaccines. This will be the crucial question in the coming weeks and constitutes ongoing work until the Update from CONTENTS MRC scientists join epidemic comes to an end.” the MRC Chief At a press conference held at NIMR on 1 May, Dr Hay Executive front line in global added: “Influenza viruses, such as the swine-like human 03 Update from the MRC Chief Executive influenza A (H1N1), can mutate rapidly. It’s therefore important that we monitor the characteristics of the virus April saw the Government fight against flu announce the budget 04 £7m investment to as it may spread around the globe. We need to monitor underpin genetics and for changes in its sensitivity to antiviral medicines and for 2009/10, and despite genomics research outbreak also see whether, like seasonal human influenza viruses, challenging economic circumstances the MRC 05 Boosting research to the virus changes such that the vaccine has to be updated. beat addiction We will be analysing the virus over the next few days and was delighted to hear that As fears of a global ’flu pandemic grabbed the the science budget ring-fence over the headlines in April and May, scientists at the MRC weeks but it will be some months before a vaccine 07 European Parliament is available.” spending period has been maintained. votes on animal research National Institute for Medical Research (NIMR) were working hard to analyse and monitor isolates of the In the meantime, the UK is well prepared should the We are confident this will allow us to honour all 08 Industry update existing grants and contracts from our generous virus, known as influenza A (H1N1), to help develop population start to become ill. -

Bzhreport2010web.Pdf
2008–2010 REPORT REPORT RESEARCH AND EDUCATION IN MOLECULAR LIFE SCIENCESRESEARCH AND EDUCATION RESEARCH AND EDUCATION IN MOLECULAR LIFE SCIENCES HEIDELBERG UNIVERSITY BIOCHEMISTRY CENTER REPORT 2008–2010 Biochemie-Zentrum der Universität Heidelberg der Universität Biochemie-Zentrum BZH_Report_U1_RZ.indd 3 01.03.2011 10:26:40 Uhr Biochemie-Zentrum der Universität Heidelberg (BZH) Im Neuenheimer Feld 328 D-69120 Heidelberg Germany Phone: +49 (0)6221 54 4154 Fax: +49 (0)6221 54 5356 www.bzh.uni-heidelberg.de Director: Prof. Dr. Michael Brunner Editors: Prof. Dr. Irmgard Sinning Dipl.-Kfr. Catarina Vill-Härtlein Layout: Dipl.-Kfr. Catarina Vill-Härtlein Cover: Dipl.-Grafik-Designerin Anke Heinzelmann For a copy of this report please contact: Barbara Bohne (BZH-Administration) e-mail: [email protected] Introduction 4 Research Groups Michael Brunner 6 Elisabeth Davioud-Charvet 10 Tamás Fischer 12 Ed Hurt 14 Wilhelm Just 18 Martin Koš 20 Luise Krauth-Siegel 22 Johannes Lechner 24 Dimitris Liakopoulos 26 Walter Nickel 28 Heiner Schirmer 30 Irmgard Sinning 32 Thomas Söllner 36 Frank Weber 38 Felix Wieland / Britta Brügger 40 Teaching at the BZH 44 Facilities 46 Funding 50 Theses 54 Publications 56 Staff 64 Scientific Advisory Board 68 How to get to the BZH 71 Welcome to the BZH! Virtually all cellular functions are maintained by biological machines, which consist of macromolecular proteinaceous assemblies and nucleic acid/protein particles. The biogenesis and structure of such mo- lecular machines, as well as their function, -

NIMR) C.1960–C.2000
TECHNOLOGY, TECHNIQUES, AND TECHNICIANS AT THE NATIONAL INSTITUTE FOR MEDICAL RESEARCH (NIMR) c.1960–c.2000 The transcript of a Witness Seminar held by the History of Modern Biomedicine Research Group, Queen Mary University of London, on 17 June 2014 Edited by C Overy and E M Tansey Volume 59 2016 ©The Trustee of the Wellcome Trust, London, 2016 First published by Queen Mary University of London, 2016 The History of Modern Biomedicine Research Group is funded by the Wellcome Trust, which is a registered charity, no. 210183. ISBN 978 1 91019 5161 All volumes are freely available online at www.histmodbiomed.org Please cite as: Overy C, Tansey E M. (eds) (2016) Technology, Techniques, and Technicians at the National Institute for Medical Research (NIMR) c.1960–c.2000. Wellcome Witnesses to Contemporary Medicine, vol. 59. London: Queen Mary University of London. CONTENTS What is a Witness Seminar? v Acknowledgements E M Tansey and C Overy vii Illustrations and credits ix Abbreviations xv Introduction Jim Smith xvii Transcript Edited by C Overy and E M Tansey 1 Appendix 1 Floor plans of the NIMR at Holly Hill 125 Appendix 2 The fraction collector 127 Appendix 3 A history of the chemistry laboratory: Form and function Peter J T Morris 129 Appendix 4 Computers at the NIMR Steven White 145 Appendix 5 The planimeter Anthony S Travis 149 Appendix 6 The first decade at the NIMR, Mill Hill: The instruments that revolutionized analytical chemistry Anthony S Travis 151 Appendix 7 Apparatus used to prepare cell walls Ian Mathison 171 Appendix 8 References by Sutherland et al. -

Nobel Prize in Chemistry 2014 for Stefan Hell Unraveling the True
alumni 2015 Cover Story Nobel Prize in Chemistry 2014 for Stefan Hell Research Reports Unraveling the true identity of the brain of Carl Friedrich Gauss News from the institute Patrick Cramer and Melina Schuh appointed as new Directors INHALT / CONTENTS Nobelpreis für Chemie 2014 für Stefan Hell / Nobel Prize in Chemistry 2014 for Stefan Hell 5 Ein großer Tag am Institut – Stefan Hell erhält den Chemie-Nobelpreis / A great day at the institute – Stefan Hell receives Nobel Prize in Chemistry 10 Lichtblicke in die Nanowelt / Nanoscopy with focused light 12 Die Nobelpreis-Verleihung in Stockholm / The Nobel Award Ceremony in Stockholm 14 Helle Willkommensfreude und eine gelungene Überraschung / A hell of a welcome at the institute 16 Nobelpreisträgertagung in Lindau / Nobel Laureate Meeting in Lindau Berichte aus der Wissenschaft / Research reports 18 Gezinkte Gegenwehr im Kampf gegen Krankheitserreger / Scientists unveil secrets of an important natural antibiotic 21 Forscher bremsen Parkinson bei Mäusen aus / Putting the brakes on Parkinson’s disease 24 Live aus dem Hühnerei / Live from the hen’s egg or sure the outstanding event of the recent past at our story behind their discovery and how they had established the 28 Wahre Identität des Gauß-Gehirns aufgeklärt / Unraveling the true identity of the brain of Carl Friedrich Gauss institute was the announcement of Stefan Hell as winner method now routinely used in laboratories all over the world. 32 Eine Methode schreibt Geschichte / A method makes history of the Nobel Prize in Chemistry on October 8th 2014. Two symposia honoring Nobel Laureate Erwin Neher, who 36 Schärferer Blick in die Proteinfabrik als je zuvor / Sharper view into the protein factory than ever before FWhat a great success for Stefan Hell, for the MPI-BPC, and had his 70th birthday in 2014, were held bringing together com- for the Max Planck Society! The weeks and months following panions, current and former collegues, as well as coworkers.