Curriculum Vitae Professor Dr. Patrick Cramer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
BSCB Newsletter 2017D
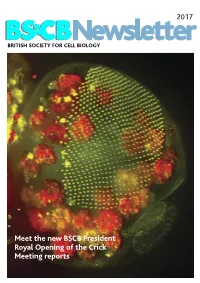
2017 BSCB Newsletter BRITISH SOCIETY FOR CELL BIOLOGY Meet the new BSCB President Royal Opening of the Crick Meeting reports 2017 CONTENTS BSCB Newsletter News 2 Book reviews 7 Features 8 Meeting Reports 24 Summer students 30 Society Business 33 Editorial Welcome to the 2017 BSCB newsletter. After several meeting hosted several well received events for our Front cover: years of excellent service, Kate Nobes has stepped PhD and Postdoc members, which we discuss on The head of a Drosophila pupa. The developing down and handed the reins over to me. I’ve enjoyed page 5. Our PhD and Postdoc reps are working hard compound eye (green) is putting together this years’ newsletter. It’s been great to make the event bigger and better for next year! The composed of several hundred simple units called ommatidia to hear what our members have been up to, and I social events were well attended including the now arranged in an extremely hope you will enjoy reading it. infamous annual “Pub Quiz” and disco after the regular array. The giant conference dinner. Members will be relieved to know polyploidy cells of the fat body (red), the fly equivalent of the The 2016 BSCB/DB spring meeting, organised by our we aren’t including any photos from that here. mammalian liver and adipose committee members Buzz Baum (UCL), Silke tissue, occupy a big area of the Robatzek and Steve Royle, had a particular focus on In this issue, we highlight the great work the BSCB head. Cells and Tissue Architecture, Growth & Cell Division, has been doing to engage young scientists. -

Curriculum Vitae Prof. Dr. Geoffrey L. Smith
Curriculum Vitae Prof. Dr. Geoffrey L. Smith Name: Geoffrey L. Smith Born: 23 July 1955 Main areas of research: Microbiology, virology, immunology, vaccinia virus, vaccines Geoffrey L. Smith is a British microbiologist and virologist specialising in the area of poxviruses. His research examines the interaction of poxviruses with the infected host cell and the immune system, concentrating especially on the vaccinia virus that was the vaccine used to eradicate smallpox. His research has enabled new vaccination concepts to be developed and provided important insights into how viruses evade the host innate immune response and cause disease. Academic and Professional Career since 2011 Chair of the Pathology Department, University of Cambridge, UK and Principal Research Fellow, Wellcome Trust 2000 ‐ 2011 Director, Department of Virology, Imperial College London, UK 1989 ‐ 2000 Reader then Professor, University of Oxford, UK 1985 ‐ 1989 Lecturer in Virology, University of Cambridge, UK 1981 ‐ 1984 Postdoctoral Fellow, National Institutes of Health, Bethesda, USA 1981 PhD, National Institute for Medical Research, Mill Hill, London, UK Functions in Scientific Societies and Committees since 2015 Chair of the scientific council, Friedrich Löffler Institute 2012 ‐ 2016 Member, Biomedical Panel of the University Research Grant Committee, Hong Kong 2011 ‐ 2014 President, International Union of Microbiological Societies 2009 ‐ 2012 Chair, Royal Society Committee for Scientific Aspects of International Security Nationale Akademie der Wissenschaften Leopoldina -

Roger D. Kornberg Stanford University, School of Medicin, Fairchild D 123, Stanford, CA 94305-5126, USA
THE MOLECULAR BASIS OF EUKARYOTIC TRANSCRIPTION Nobel Lecture, December 8, 2006 by Roger D. Kornberg Stanford University, School of Medicin, Fairchild D 123, Stanford, CA 94305-5126, USA. I am deeply grateful for the honor bestowed on me by the Nobel Committee for Chemistry and the Royal Swedish Academy of Sciences. It is an honor I share with my collaborators. It is also recognition of the many who have con- tributed over the past quarter century to the study of transcription. THE NUCLEOSOME My own involvement in studies of transcription began with the dis- covery of the nucleosome, the basic unit of DNA coiling in eukaryote chromosomes [1]. X-ray studies and protein chemistry led me to propose the wrapping of DNA around a set of eight histone molecules in the nucleosome (Fig. 1). Some years later, Yahli Lorch and I found this wrapping of DNA prevents the ini- tiation of transcription in vitro [2]. Michael Grunstein and colleagues showed nucleosomes interfere with transcription in vivo [3]. The nuc- leosome serves as a general gene repressor. It assures the inactivity of all the many thousands of genes in eukaryotic cells except those Figure 1. The nucleosome, fundamental whose transcription is brought particle of the eukaryote chromosome. about by specific positive regulatory Schematic shows the coiling of DNA around mechanisms. What are these posi- a set of eight histones in the nucleosome, tive regulatory mechanisms? How is the further coiling in condensed (transcrip- tionally inactive) chromatin, and uncoiling repression by the nucleosome over- for interaction with the RNA polymerase II come for transcription? Our recent (pol II) transcription machinery. -

The Cramer Laboratory Unraveled the Structural Basis of Eukaryotic Gene Transcription and Cellular Mechanisms of Gene Regulation
The Cramer laboratory unraveled the structural basis of eukaryotic gene transcription and cellular mechanisms of gene regulation. Patrick Cramer determined the first structure of a eukaryotic RNA polymerase, Pol II, with Roger Kornberg (Cramer, Science 2001). Over the last two decades, the Cramer laboratory used a combination of crystallography, cryo-EM, and chemical crosslinking to determine structures of Pol II in many functional complexes (reviewed in Osman, Annu Rev Cell Dev Biol 2020). These include the Pol II pre-initiation complex with the coactivator Mediator, a 46-protein assembly that provides the basis for transcription initiation and regulation. The Cramer lab also developed methods for integrated structural biology of macromolecular assemblies and for functional multi- omics. They established biological concepts such as the tunability of the polymerase active site (Kettenberger, Cell 2003), indirect promoter recognition (Engel, Nature 2013), or elongation-limited initiation regulation of genes (Gressel, eLife 2017). Recently, the Cramer lab reported structures of mammalian Pol II (Bernecky, Nature 2016) and paused and activated complexes (Vos et al., Nature 2018a, 2018b). This elucidated the mechanisms of transcriptional regulation during elongation. Cramer further pioneered structural studies of alternative RNA polymerases. They recently solved the structure of the polymerase of the coronavirus SARS-CoV-2 (Hillen, Nature 2020) and clarified the mechanism of the polymerase inhibitor remdesivir, the only FDA-approved drug for COVID-19 (Kokic, BioRxiv 2020). The Cramer group also resolved first structures of Pol I (Engel, Nature 2013) and the mitochondrial RNA polymerase (Ringel, Nature 2011), and initiation and elongation complexes for both (Engel, Cell 2017; Hillen Cell 2017). -

Brave New World View .Com Last Month, ∼200 Scientists Arrived at the EMBL in Heidelberg, Germany to Attend the Millennium Symposium on Structural Biology*
© 2000 Nature America Inc. • http://structbio.nature.com editorial may 2000 vol. 7 no. 5 molecular form & function Brave new world view .com Last month, ∼200 scientists arrived at the EMBL in Heidelberg, Germany to attend the Millennium Symposium on Structural Biology*. Although the word ‘millennium’ has been overused recently, this symposium did, in fact, earn the right to use that term, which is synony- mous with looking to the future. An underlying theme of the conference was cutting edge tech- nology. Researchers now have a large number of tools at their disposal to analyze biological molecules, and this meeting — more than many others in recent memory — made this apparent. The sessions were organized around some of the most exciting current research in molecular and http://structbio.nature • structural biology: nucleic acid–protein interactions, transport across membranes and within cells, motor proteins and the cytoskeleton, catalysis, and future challenges in structural biology. All were informed by results from a variety of techniques. Here are a few of the highlights. Big structures and conformational changes Francis Tsai (Yale University), one of Paul Sigler’s postdoctoral associates, began the first session with a tribute to Sigler, who died earlier this year. His talk focused on how the large eukaryotic initiation complex determines the correct orientation on the DNA, so that transcription pro- ceeds in the proper direction. The same session continued with presentations of some long- awaited structures — of prokaryotic core RNA polymerase (Seth Darst, The Rockefeller 2000 Nature America Inc. © University) and eukaryotic RNA polymerase II (Patrick Cramer, Stanford University) — both of which should help to answer additional questions about the mechanisms of gene expression. -

Curriculum Vitae Prof. Dr. Patrick Cramer
Curriculum Vitae Prof. Dr. Patrick Cramer Name: Patrick Cramer Geboren: 3. Februar 1963 Foto: MPI für Biophysikalische Chemie Forschungsschwerpunkte: Molekularbiologie, Gentranskription, Genregulation, RNA-Polymerasen, dreidimensionale Struktur von Enzymen Patrick Cramer ist Chemiker und Molekularbiologe. Er erforscht, wie Zellen im Erbgut gespeicherte Informationen auslesen (Gentranskription). Er konnte die dreidimensionale Struktur eines der größten Enzyme im Zellkern entschlüsseln und dadurch wesentliche Teile des Transkriptions-Mechanismus aufklären. Akademischer und beruflicher Werdegang seit 2017 Honorarprofessor, Georg-August-Universität Göttingen seit 2014 Direktor, Max-Planck-Institut für Biophysikalische Chemie, Göttingen 2010 - 2013 Leiter, Abteilung Biochemie, Ludwig-Maximilians-Universität (LMU) München 2004 - 2013 Direktor, Genzentrum München, LMU München 2004 - 2014 Professor für Biochemie, LMU München 2001 - 2003 Tenure-Track Professor für Biochemie, LMU München 1999 - 2001 Postdoktorand, Stanford University, Stanford, USA 1998 Promotion in Biochemie, Universität Heidelberg/EMBL Grenoble, Frankreich 1995 - 1998 Doktorand, EMBL Grenoble, Frankreich 1994 Diplom in Chemie, Universität Heidelberg 1989 - 1995 Studium der Chemie, Universitäten Stuttgart, Heidelberg, Bristol und Cambridge, UK Nationale Akademie der Wissenschaften Leopoldina www.leopoldina.org 1 Funktionen in wissenschaftlichen Gesellschaften und Gremien seit 2017 Koordinator, International Max Planck Research School for Genome Science seit 2017 Mitglied, Advisory -

Structural Basis of Transcription: -Amanitin–RNA Polymerase II
Structural basis of transcription: ␣-Amanitin–RNA polymerase II cocrystal at 2.8 Å resolution David A. Bushnell, Patrick Cramer*, and Roger D. Kornberg† Department of Structural Biology, Stanford University School of Medicine, Stanford, CA 94305-5126 Contributed by Roger D. Kornberg, December 12, 2001 The structure of RNA polymerase II in a complex with the inhibitor Table 1. Crystallographic data ␣-amanitin has been determined by x-ray crystallography. The Space group I222 structure of the complex indicates the likely basis of inhibition and Unit cell, Å 122.5 by 222.5 by 374.2 gives unexpected insight into the transcription mechanism. Wavelength, Å 0.965 Mosaicity, ° 0.44 he structure of 10-subunit 0.5-MDa yeast RNA polymerase Resolution, Å 20–2.8 (2.9–2.8) TII (pol II), recently determined at 2.8 Å resolution, reveals Completeness, % 99.8 (99.4) the architecture and key functional elements of the enzyme (1). Redundancy 3.9 (2.9) The two largest subunits, Rpb1 and Rpb2, lie at the center, on Unique reflections 124,441 (12,292) either side of a nucleic acid-binding cleft, with the many smaller Rsym,% 6.7 (21.6) subunits arrayed around the outside. Rpb1 and Rpb2 interact ϭ extensively in the region of the active site and also through a Values in parentheses correspond to the highest-resolution shell. Rsym ⌺ ͉I(i,h) Ϫ͗I(h)͉͘͞⌺ ͉I(i,h)͉, where ͗I(h)͘ is the mean of the I observations of domain of Rpb1 that lies on the Rpb2 side of the cleft, connected i,h i,h ␣ reflection h. -

Breakthrough How Ideas Exchanged in the Canteen Can Lead to Research Discoveries
News from the Medical Research Council network Summer 2013 100 years of life-changing discoveries The greatcoffee breakthrough How ideas exchanged in the canteen can lead to research discoveries. Discoveries of the century Well-known figures give us their thoughts on the biggest medical advances, past and future. Network can also be downloaded as a PDF at: www.mrc.ac.uk/network CONTENTS NEWS News COMMENT FROM A phenomenal legacy begins 3 A phenomenal John Informatics research centres launched across the UK 3 legacy begins Speedier access to brain tissue for research 5 Savill Universities and Science Minister David Willetts has officially opened the MRC-NIHR Phenome Centre CHIEF EXECUTIVE at Imperial College London, which will re-purpose some of the sophisticated technology from the Royal opening for new LMB building 5 The launch of four e-health London 2012 Olympics Anti-Doping Centre. informatics research centres (eHIRCs) and the UK informatics Unlike our genome, which collectively describes a person’s genetic material, the phenome describes all the other chemistry of our research network by the Science body. It is the product of how our genes and environment interact throughout development and life, and is analysed by linking our Minister in May (see opposite), chemical, metabolic and physiological features and responses. By measuring phenome patterns throughout life, researchers at the Latest discoveries marked part of a £90m total centre aim to identify the patterns which separate those who develop particular diseases from those who don’t. investment in so-called big data or Gout drug offers hope for heart disease patients 10 medical bioinformatics research– something we excel at here in The centre’s first three pilot studies are now underway. -

(12) Patent Application Publication (10) Pub. No.: US 2003/0232369 A1 Bushnell Et Al
US 2003O232369A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0232369 A1 Bushnell et al. (43) Pub. Date: Dec. 18, 2003 (54) MOLECULAR STRUCTURE OF RNA Publication Classification POLYMERASE II (51) Int. Cl." ............................ C12O 1/68; G06F 19/00; (76) Inventors: David A. Bushnell, Menlo Park, CA G01N 33/48; G01N 33/50 (US); Roger D. Kornberg, Atherton, (52) U.S. Cl. ................................................... 435/6; 702/20 CA (US); Patrick Cramer, Munich (DE) (57) ABSTRACT Correspondence Address: Crystals and structures are provided for an eukaryotic RNA BOZICEVIC, FIELD & FRANCIS LLP polymerase, and an elongation complex containing a 200 MIDDLEFIELD RID eukaryotic RNA polymerase. The Structures and structural coordinates are useful in Structural homology deduction, in SUTE 200 developing and Screening agents that affect the activity of MENLO PARK, CA 94025 (US) eukaryotic RNA polymerase, and in designing modified Appl. No.: 10/418,772 forms of eukaryotic RNA polymerase. The structure infor (21) mation may be provided in a computer readable form, e.g. (22) Filed: Apr. 17, 2003 as a database of atomic coordinates, or as a three-dimen Sional model. The Structures are useful, for example, in Related U.S. Application Data modeling interactions of the enzyme with DNA, RNA, transcription factors, nucleotides, etc. The Structures are also (60) Provisional application No. 60/373,486, filed on Apr. used to identify molecules that bind to or otherwise interact 17, 2002. with Structural elements in the polymerase. Rpb1 active site loop Rpb1 (133-c.35 Rpb1 it 3i 1 Patent Application Publication Dec. 18, 2003 Sheet 1 of 26 US 2003/0232369 A1 FIGURE 1 Rpb1 active site loop A. -

EMBO Facts & Figures
excellence in life sciences young investigators|courses,workshops,conference series & symposia|installation grantees|long-term fellows|short-term fellows|policy, science & society|the EMBO Journal|EMBO reports|molecular systems biology|EMBO molecular medicine|global exchange|gold medal|the EMBO meeting|women in science| EMBO reports|molecular systems biology|EMBO molecular medicine|global exchange|gold medal|the EMBO meeting|women in science|young investigators|courses,workshops,conference series & symposia|installation grantees|long-term fellows|short-term fellows|policy, science & society|the EMBO Journal| global exchange|gold medal|the EMBO meeting|women in science|young investigators|long-term fellows|short-term fellows|policy, science & society|the EMBO Journal|courses,workshops,conference series & symposia|EMBO reports|molecular systems biology|EMBO molecular medicine|installation grantees| EMBO molecular medicine|installation grantees|long-term fellows|gold medal|molecular systems biology|short-term fellows|the EMBO meeting|womenReykjavik in science|young investigators|courses,workshops,conference series & symposia|global exchange|EMBO reports|policy, science & society|the EMBO Journal| gold medal|the EMBO meeting|women in science|young investigators|courses,workshops,conference series & symposia|global exchange|policy, science & society|the EMBO Journal|EMBO reports|molecular systems biology|EMBO molecular medicine|installation grantees|long-term fellows|short-term fellows| courses,workshops,conference series & symposia|global -

Native Molecule Sequencing by Nano-ID Reveals Synthesis and Stability of RNA Isoforms
Downloaded from genome.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Method Native molecule sequencing by nano-ID reveals synthesis and stability of RNA isoforms Kerstin C. Maier,1 Saskia Gressel, Patrick Cramer, and Björn Schwalb1 Department of Molecular Biology, Max-Planck-Institute for Biophysical Chemistry, 37077 Göttingen, Germany Eukaryotic genes often generate a variety of RNA isoforms that can lead to functionally distinct protein variants. The syn- thesis and stability of RNA isoforms is poorly characterized because current methods to quantify RNA metabolism use short-read sequencing and cannot detect RNA isoforms. Here we present nanopore sequencing–based isoform dynamics (nano-ID), a method that detects newly synthesized RNA isoforms and monitors isoform metabolism. Nano-ID combines metabolic RNA labeling, long-read nanopore sequencing of native RNA molecules, and machine learning. Nano-ID derives RNA stability estimates and evaluates stability determining factors such as RNA sequence, poly(A)-tail length, secondary structure, translation efficiency, and RNA-binding proteins. Application of nano-ID to the heat shock response in human cells reveals that many RNA isoforms change their stability. Nano-ID also shows that the metabolism of individual RNA isoforms differs strongly from that estimated for the combined RNA signal at a specific gene locus. Nano-ID enables studies of RNA metabolism at the level of single RNA molecules and isoforms in different cell states and conditions. [Supplemental material is available for this article.] In metazoan cells, a single gene locus can give rise to a variety of erally cannot be assigned to RNA isoforms. -

Jubiläum 25 Jahre Forschungspreis: Auszeichnungen Und Kommunikation Im Dienste Der Wissenschaft Zwischen Gesellschaft, Wirtschaft Und Politik
Jubiläum 25 Jahre Forschungspreis: Auszeichnungen und Kommunikation im Dienste der Wissenschaft zwischen Gesellschaft, Wirtschaft und Politik Preisträger Prof. Dr. Immanuel Bloch Quantenoptik: Atome in Gittern aus Licht Prof. Dr. Axel Ockenfels Verhaltensökonomie: Die Vermessung der Menschlichkeit Prof. Dr. Patrick Cramer Biochemie: Großes Kino aus der Zelle Prof. Dr. Sebastian Conrad Geschichtswissenschaft: Die Vielfalt der Moderne LEHMEHJÅ?D>7BJ VORWORT Sehr geehrte Damen und Herren, der Wandel ist die große Gewissheit. Der konstruk- tive Umgang mit Veränderung und die Fähigkeit, das Neue zu gestalten, sind zu zentralen Kompetenzen dieser Tage aufgestiegen. Um das Neue jedoch tat- sächlich gestalten zu können, müssen wir unsere Aufmerksamkeit den Randbereichen widmen. Wir müssen Expeditionen unterneh- men und das Gespräch mit denen suchen, die jeden Tag die große und die kleine Welt des Wissens neu kartieren. Seit 25 Jahren führt der Forschungspreis der Philip Morris Stiftung diesen Dialog, indem er führende Wissenschaftler auszeichnet und sie im Lichte der Öffentlichkeit preist. Willkommen im Wandel! :;JJC7H:;B8EI Kdgh^ioZcYZgYZh@jgVidg^jbhYZgE]^a^eBdgg^hHi^[ijc\ INHALT 25 JAHRE FORSCHUNGSPREIS Hochkarätige Juryarbeit – an 365 Tagen im Jahr ..........................................................................24 Essay: Erkenntnisgewinne für ein neues Weltbewusstsein .........................................................28 Der Forschungspreis im Wandel der Zeit ....................................................................................52