Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside Or Colistin Therapy: a Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyponatremia and Hypernatremia MICHAEL M
This is a corrected version of the article that appeared in print. Diagnosis and Management of Sodium Disorders: Hyponatremia and Hypernatremia MICHAEL M. BRAUN, DO, Madigan Army Medical Center, Tacoma, Washington CRAIG H. BARSTOW, MD, Womack Army Medical Center, Fort Bragg, North Carolina NATASHA J. PYZOCHA, DO, Madigan Army Medical Center, Tacoma, Washington Hyponatremia and hypernatremia are common findings in the inpatient and outpatient settings. Sodium disorders are associated with an increased risk of morbidity and mortality. Plasma osmolality plays a critical role in the patho- physiology and treatment of sodium disorders. Hyponatremia and hypernatremia are classified based on volume status (hypovolemia, euvolemia, and hypervolemia). Sodium disorders are diagnosed by findings from the history, physical examination, laboratory studies, and evaluation of volume status. Treatment is based on symptoms and underlying causes. In general, hyponatremia is treated with fluid restriction (in the setting of euvolemia), isotonic saline (in hypovolemia), and diuresis (in hypervolemia). A combination of these therapies may be needed based on the presentation. Hypertonic saline is used to treat severe symptomatic hyponatremia. Medications such as vaptans may have a role in the treatment of euvolemic and hypervolemic hyponatremia. The treatment of hypernatremia involves correcting the underlying cause and correcting the free water deficit. Am( Fam Physician. 2015;91(5):299-307. Copy- right © 2015 American Academy of Family Physicians.) More online yponatremia is a common elec- a worse prognosis in patients with liver cir- at http://www. trolyte disorder defined as a rhosis, pulmonary hypertension, myocardial aafp.org/afp. serum sodium level of less than infarction, chronic kidney disease, hip frac- CME This clinical content 135 mEq per L.1-3 A Dutch sys- tures, and pulmonary embolism.1,8-10 conforms to AAFP criteria Htematic review of 53 studies showed that the for continuing medical Etiology and Pathophysiology education (CME). -

Hypokalaemia in a Woman with Eating Disorder
Grand Rounds Vol 11 pages 53–55 Specialities: Acute Medicine; Nephrology; Psychiatry Article Type: Case Report DOI: 10.1102/1470-5206.2011.0013 ß 2011 e-MED Ltd Hypokalaemia in a woman with eating disorder Zachary Z. Brenera, Boris Medvedovskya, James F. Winchestera and Michael Bergmanb aDivision of Nephrology, Department of Medicine, Beth Israel Medical Center, Albert Einstein School of Medicine of Yeshiva University, New York, USA; bDepartment of Medicine, Campus Golda, Rabin Medical Center, Petah-Tikva, Tel-Aviv University, Israel Corresponding address: Dr Zachary Z. Brener, 350 E. 17th St., Division of Nephrology, Beth Israel Medical Center, New York, NY 10003, USA. Email: [email protected] Date accepted for publication 13 April 2011 Abstract Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. A concealed diuretic abuse should be suspected, especially in young women with eating disorders. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to early and accurate diagnosis. Keywords Hypokalaemia; eating disorders; diuretics. Introduction Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. After the exclusion of the most obvious causes, a concealed diuretic abuse associated with or without surreptitious vomiting and laxative abuse should be suspected, especially in young women concerned with their body image. A conclusive diagnosis may be difficult as such patients often vigorously deny diuretic intake[1]. Also, only a minority of patients with eating disorders (approximately 6%) abuse diuretics[2–4]. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to an early and accurate diagnosis. -

Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
medical sciences Review Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia Faheemuddin Ahmed 1,* and Abdul Mohammed 2 1 OSF Saint Anthony Medical Center, 5666 E State St, Rockford, IL 61108, USA 2 Advocate Illinois Masonic Medical Center, 833 W Wellington Ave, Chicago, IL 60657, USA; [email protected] * Correspondence: [email protected] Received: 20 February 2019; Accepted: 2 April 2019; Published: 4 April 2019 Abstract: Magnesium is the fourth most abundant cation in the body and the second most abundant intracellular cation. It plays an important role in different organ systems at the cellular and enzymatic levels. Despite its importance, it still has not received the needed attention either in the medical literature or in clinical practice in comparison to other electrolytes like sodium, potassium, and calcium. Hypomagnesemia can lead to many clinical manifestations with some being life-threatening. The reported incidence is less likely than expected in the general population. We present a comprehensive review of different aspects of magnesium physiology and hypomagnesemia which can help clinicians in understanding, identifying, and treating this disorder. Keywords: magnesium; proton pump inhibitors; diuretics; hypomagnesemia 1. Introduction Magnesium is one of the most abundant cation in the body as well as an abundant intracellular cation. It plays an important role in molecular, biochemical, physiological, and pharmacological functions in the body. The importance of magnesium is well known, but still it is the forgotten electrolyte. The reason for it not getting the needed attention is because of rare symptomatology until levels are really low and also because of a lack of proper understanding of magnesium physiology. -

Novel Mutations Associated with Inherited Human Calcium-Sensing
1 180 A García-Castaño, Novel mutations in the calcium 180:1 59–70 Clinical Study L Madariaga and others receptor gene Novel mutations associated with inherited human calcium-sensing receptor disorders: A clinical genetic study Alejandro García-Castaño1,*, Leire Madariaga1,2,*, Gustavo Pérez de Nanclares1,2, Gema Ariceta3, Sonia Gaztambide1,2 and Luis Castaño1,2 on behalf of Spanish Endocrinology Group and Renal Tube Group Correspondence should be addressed 1Biocruces Bizkaia Health Research Institute, CIBERDEM, CIBERER, Barakaldo, Spain, 2Hospital Universitario Cruces, to L Castaño UPV/EHU, Barakaldo, Spain, and 3Hospital Universitario Materno-Infantil Vall d’Hebron, Autonomous University of Email Barcelona, Barcelona, Spain LUISANTONIO. *(A García-Castaño and L Madariaga contributed equally to this work) CASTANOGONZALEZ@ osakidetza.eus Abstract Objective: Molecular diagnosis is a useful diagnostic tool in calcium metabolism disorders. The calcium-sensing receptor (CaSR) is known to play a central role in the regulation of extracellular calcium homeostasis. We performed clinical, biochemical and genetic characterization of sequence anomalies in this receptor in a cohort of 130 individuals from 82 families with suspected alterations in the CASR gene, one of the largest series described. Methods: The CASR gene was screened for mutations by polymerase chain reaction followed by direct Sanger sequencing. Results: Presumed CaSR-inactivating mutations were found in 65 patients from 26 families. These patients had hypercalcemia (median: 11.3 mg/dL) but normal or abnormally high parathyroid hormone (PTH) levels (median: 52 pg/ mL). On the other hand, presumed CaSR-activating mutations were detected in 17 patients from eight families. These patients had a median serum calcium level of 7.4 mg/dL and hypoparathyroidism (median: PTH 13 pg/mL). -
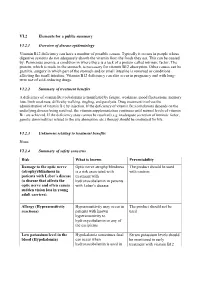
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Vitamin B12 Deficiency Can Have a Number of Possible
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vitamin B12 deficiency can have a number of possible causes. Typically it occurs in people whose digestive systems do not adequately absorb the vitamin from the foods they eat. This can be caused by: Pernicious anemia, a condition in where there is a lack of a protein called intrinsic factor. The protein, which is made in the stomach, is necessary for vitamin B12 absorption. Other causes can be gastritis, surgery in which part of the stomach and/or small intestine is removed or conditions affecting the small intestine. Vitamin B12 deficiency can also occur in pregnancy and with long- term use of acid-reducing drugs. VI.2.2 Summary of treatment benefits A deficiency of vitamin B12 (cobalamin) is manifested by fatigue, weakness, mood fluctuations, memory loss, limb weakness, difficulty walking, tingling, and paralysis. Drug treatment involves the administration of vitamin B12 by injection. If the deficiency of vitamin B12 (cobalamin) depends on the underlying disease being resolved, the vitamin supplementation continues until normal levels of vitamin B12 are achieved. If the deficiency state cannot be resolved (e.g. inadequate secretion of intrinsic factor, genetic abnormalities related to the site absorption, etc.) therapy should be continued for life. VI.2.3 Unknowns relating to treatment benefits None. VI.2.4 Summary of safety concerns Risk What is known Preventability Damage to the optic nerve Optic nerve atrophy/blindness The product should be used (atrophy)/blindness in is a risk associated with with caution. patients with Leber´s disease treatment with (a disease that affects the hydroxocobalamin in patients optic nerve and often causes with Leber´s disease. -

Mechanism of Hypokalemia in Magnesium Deficiency
SCIENCE IN RENAL MEDICINE www.jasn.org Mechanism of Hypokalemia in Magnesium Deficiency Chou-Long Huang*† and Elizabeth Kuo* *Department of Medicine, †Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center, Dallas, Texas ABSTRACT deficiency is likely associated with en- ϩ Magnesium deficiency is frequently associated with hypokalemia. Concomitant hanced renal K excretion. To support magnesium deficiency aggravates hypokalemia and renders it refractory to treat- this idea, Baehler et al.5 showed that ad- ment by potassium. Herein is reviewed literature suggesting that magnesium ministration of magnesium decreases ϩ deficiency exacerbates potassium wasting by increasing distal potassium secre- urinary K excretion and increases se- ϩ tion. A decrease in intracellular magnesium, caused by magnesium deficiency, rum K levels in a patient with Bartter releases the magnesium-mediated inhibition of ROMK channels and increases disease with combined hypomagnesemia potassium secretion. Magnesium deficiency alone, however, does not necessarily and hypokalemia. Similarly, magnesium ϩ cause hypokalemia. An increase in distal sodium delivery or elevated aldosterone replacement alone (without K ) in- ϩ levels may be required for exacerbating potassium wasting in magnesium creases serum K levels in individuals deficiency. who have hypokalemia and hypomag- nesemia and receive thiazide treatment.6 J Am Soc Nephrol 18: 2649–2652, 2007. doi: 10.1681/ASN.2007070792 Magnesium administration decreased urinary Kϩ excretion in these individuals (Dr. Charles Pak, personal communica- Hypokalemia is among the most fre- cin B, cisplatin, etc. Concomitant magne- tion, UT Southwestern Medical Center quently encountered fluid and electro- sium deficiency has long been appreci- at Dallas, July 13, 2007). -

Hyperchloremia – Why and How
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):347–353 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Brief review Hyperchloremia – Why and how Glenn T. Nagami Nephrology Section, Department of Medicine, VA Greater Los Angeles Healthcare System and David Geffen School of Medicine at UCLA, United States a r t i c l e i n f o a b s t r a c t Article history: Hyperchloremia is a common electrolyte disorder that is associated with a diverse group of Received 5 April 2016 clinical conditions. The kidney plays an important role in the regulation of chloride concen- Accepted 11 April 2016 tration through a variety of transporters that are present along the nephron. Nevertheless, Available online 3 June 2016 hyperchloremia can occur when water losses exceed sodium and chloride losses, when the capacity to handle excessive chloride is overwhelmed, or when the serum bicarbonate is low Keywords: with a concomitant rise in chloride as occurs with a normal anion gap metabolic acidosis Hyperchloremia or respiratory alkalosis. The varied nature of the underlying causes of the hyperchloremia Electrolyte disorder will, to a large extent, determine how to treat this electrolyte disturbance. Serum bicarbonate Published by Elsevier Espana,˜ S.L.U. on behalf of Sociedad Espanola˜ de Nefrologıa.´ This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/). -

An Infant with Chronic Hypernatremia
European Journal of Endocrinology (2006) 155 S141–S144 ISSN 0804-4643 An infant with chronic hypernatremia M L Marcovecchio Department of Paediatrics, University of Chieti, Via dei Vestini 5, 66100 Chieti, Italy (Correspondence should be addressed to M L Marcovecchio; Email: [email protected]) Abstract A 4-month-old boy was presented with failure to thrive, refusal to feed, delayed motor development, truncal hypotonia, and head lag. His plasma osmolality and sodium were significantly high, while his urine osmolality was inappropriately low and did not increase after desmopressin administration. Despite his hyperosmolality, he presented with a lack of thirst and became clearly polyuric and polydipsic only at the age of 2 years. Initial treatment with indomethacin was ineffective, while the combination of hydrochlorothiazide and amiloride was effective and well tolerated. European Journal of Endocrinology 155 S141–S144 Introduction (61 ml/kg) respectively. Other investigations including thyroid and adrenal function were normal. He was Chronic hypernatremia in young patients is generally refusing oral fluids despite being hypernatremic. the result of alterations in the mechanisms controlling A cranial magnetic resonance imaging scan excluded fluid balance (1). Excessive water loss, as in diabetes structural abnormalities in the hypophysis, hypo- insipidus, or an inadequate fluid intake, as in adipsic thalamus and surrounding area. There was no response hypernatremia, may be the underlying cause. The to 0.3 mg 1-desamino-8-D-arginine-vasopressin (DDAVP) differential diagnosis between these conditions is given intravenously (osmolality pre- 374 mosmol/kg; important in order to choose the appropriate treatment. post- 371 mosmol/kg). This suggested a tubular defect However, pitfalls in the diagnosis are often related to an causing nephrogenic diabetes insipidus (NDI). -

Is There a Relationship Between COVID-19 and Hyponatremia?
medicina Review Is There a Relationship between COVID-19 and Hyponatremia? Gina Gheorghe 1,2,†, Madalina Ilie 1,2, Simona Bungau 3,† , Anca Mihaela Pantea Stoian 4 , Nicolae Bacalbasa 5 and Camelia Cristina Diaconu 6,7,* 1 Department of Gastroenterology, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania; [email protected] (G.G.); [email protected] (M.I.) 2 Department of Gastroenterology, Clinical Emergency Hospital of Bucharest, 105402 Bucharest, Romania 3 Department of Pharmacy, Faculty of Medicine and Pharmacy, University of Oradea, 410028 Oradea, Romania; [email protected] 4 Department of Diabetes, Nutrition and Metabolic Diseases, “Carol Davila” University of Medicine and Pharmacy, 020475 Bucharest, Romania; [email protected] 5 Department of Visceral Surgery, Center of Excellence in Translational Medicine, Fundeni Clinical Institute, 022328 Bucharest, Romania; [email protected] 6 Department of Internal Medicine, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania 7 Department of Internal Medicine, Clinical Emergency Hospital of Bucharest, 105402 Bucharest, Romania * Correspondence: [email protected]; Tel.: +40-0726-377-300 † This author has equal contribution to the paper as the first author. Abstract: Nowadays, humanity faces one of the most serious health crises, the severe acute respi- ratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. The severity of coronavirus disease 2019 (COVID-19) pandemic is related to the high rate of interhuman transmission of the virus, variability of clinical presentation, and the absence of specific therapeutic methods. COVID-19 can manifest with non-specific symptoms and signs, especially among the elderly. In some cases, the clinical manifestations of hyponatremia may be the first to appear. -

A Case of Hypocalciuric Hypercalcemia Accompanying Cystic Fibrosis
J Clin Res Pediatr Endocrinol 2015;7(Suppl 2):77-92 A Case of Hypocalciuric Hypercalcemia Accompanying Cystic Fibrosis Yaşar Şen, Sevil Arı Yuca, Fuat Buğrul Blood and urine tests were done in order to find the etiology of hypercalcemia (PTH: 65.5 pg/mL, 25-hydroxy vitamin Selçuk University Faculty of Medicine, Department of Pediatric D: 43.5 ng/mL, urine Ca/Cr ratio 0.19, urine Ca clearance Endorcinology, Konya, Turkey 0.004). In the CaSR gene mutation study, A986S and R990G polymorphisms were heterozygous. 1 month after hydration Introduction: Familial hypocalciuric hypercalcemia (FHH) and clinical state being back to normal, Ca levels were is an autosomal dominant disorder which occurs by an in normal range. In stressful situations, he experienced inactivating gene mutation in the calcium (Ca)-sensing hypernatremia and hypercalcemia together. receptor gene (CaSR). Prevalence is estimated at 1: 78000. Discussion: Polymorphisms may change protein function Ca regulates parathormone (PTH) secretion via CaSR on and capacity of one to repair its damaged DNA. Genetic parathyroid cells. Low levels of Ca increases PTH secretion. polymorphisms help us to define personal sensitivities to CaSR mutation that causes loss of function, leads to total some diseases. The most common CaSR polymorphisms or partial insensitivity of parathyroid cells to Ca’s inhibitory are A986S, R990G, Q1011E and A826T. Our patient effect. For this reason, in order to suppress Ca’s PTH had A986S and R990G mutations. Heterozygous CaSR secretory effect, the setpoint is raised. A higher blood Ca gene mutations generally cause mild disorders in clinic. level is needed to suppress the PTH secretion. -
![Serum Sodium Concentration [Na+] Less Than 135 Meq/L. INCIDENCE](https://docslib.b-cdn.net/cover/6326/serum-sodium-concentration-na-less-than-135-meq-l-incidence-1356326.webp)
Serum Sodium Concentration [Na+] Less Than 135 Meq/L. INCIDENCE
HYPONATREMIA DEFINITION: Serum sodium concentration [Na+] less than 135 mEq/L. INCIDENCE IN CRITICAL ILLNESS: 30%. (The most common electrolyte abnormality encountered in clinical medicine.) ETIOLOGY: Hypo-osmolar hyponatremia: Serum osmolality is low (< 280 mOsm/kg H2O). Hypovolemic: Total body water deficit + greater degree of total body sodium deficit. o Renal losses (urine [Na+] > 20 mmol/L): Diuretic excess; mineralocorticoid deficiency; cerebral salt wasting; bicarbonaturia (renal tubular acidosis and metabolic alkalosis); ketonuria; osmotic diuresis. o Extrarenal losses (urine [Na+] < 20 mmol/L): Vomiting; diarrhea; “third spacing” (burns, pancreatitis, trauma). Euvolemic: Total body water excess + normal total body sodium. o The most common subcategory of hyponatremia. o Includes dilutional hyponatremia. Excess of water relative to sodium; serum chloride concentration is usually normal. o Includes SIADH: Diagnosis of exclusion. Inclusion criteria are plasma osmolality < 270 mOsm/kg + H2O; urine osmolality > 100 mOsm/kg H2O; euvolemia; urine [Na ] elevated; adrenal, thyroid, pituitary, renal insufficiency absent; diuretic use absent. o Urine [Na+] is typically > 20 mmol/L. o Glucocorticoid deficiency; hypothyroidism; stress; medications (vasopressin analogs, drugs that enhance vasopressin release, drugs that potentiate renal action of vasopressin, haloperidol, amitriptyline, other psychotropic medications). Hypervolemic: Total body water excess >>> total body sodium excess. o Urine [Na+] > 20 mmol/L: Renal failure. o Urine -

The Prognostic Effects of Hyponatremia and Hyperchloremia on Postoperative NSCLC Patients
Current Problems in Cancer 43 (2019) 402–410 Contents lists available at ScienceDirect Current Problems in Cancer journal homepage: www.elsevier.com/locate/cpcancer The prognostic effects of hyponatremia and hyperchloremia on postoperative NSCLC patients Wei Li a,b,1, Xiaowei Chen a,1, Liguang Wang a,c, Yu Wang a, ∗ Cuicui Huang a, Guanghui Wang a,d, Jiajun Du a,d, a Institute of Oncology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China b Department of Thoracic Surgery, Shandong Juxian People’s Hospital, Rizhao, PR China c Department of Oncology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China d Department of Thoracic Surgery, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China a b s t r a c t Electrolytic disorders are common in lung cancer patients. But the association between serum electrolytes levels and survival in patients undergoing lung cancer resections for non–small-cell lung cancer (NSCLC) has been poorly inves- tigated. A retrospective study was conducted on consecutive postoperative NSCLC patients. Pearson’s test was used to determine the association between serum sodium and chlorine levels and clinical characteristics, and cox regression and Kaplan-Meier model were applied to analyze risk factors on overall survival. We found that hyponatremia was an independent prognostic factor associated with poor prognosis in NSCLC patients undergoing complete resection (log-rank test, P = 0.004). In addition, we found that hyperchloremia predicted a poor clinical outcome in patients with non-anion-gap (log-rank test, P = 0.011), whereas it predicted a favorable clinical outcome in patients with high- anion-gap (log-rank test, P = 0.002).