Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hypokalaemia in a Woman with Eating Disorder
Grand Rounds Vol 11 pages 53–55 Specialities: Acute Medicine; Nephrology; Psychiatry Article Type: Case Report DOI: 10.1102/1470-5206.2011.0013 ß 2011 e-MED Ltd Hypokalaemia in a woman with eating disorder Zachary Z. Brenera, Boris Medvedovskya, James F. Winchestera and Michael Bergmanb aDivision of Nephrology, Department of Medicine, Beth Israel Medical Center, Albert Einstein School of Medicine of Yeshiva University, New York, USA; bDepartment of Medicine, Campus Golda, Rabin Medical Center, Petah-Tikva, Tel-Aviv University, Israel Corresponding address: Dr Zachary Z. Brener, 350 E. 17th St., Division of Nephrology, Beth Israel Medical Center, New York, NY 10003, USA. Email: [email protected] Date accepted for publication 13 April 2011 Abstract Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. A concealed diuretic abuse should be suspected, especially in young women with eating disorders. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to early and accurate diagnosis. Keywords Hypokalaemia; eating disorders; diuretics. Introduction Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. After the exclusion of the most obvious causes, a concealed diuretic abuse associated with or without surreptitious vomiting and laxative abuse should be suspected, especially in young women concerned with their body image. A conclusive diagnosis may be difficult as such patients often vigorously deny diuretic intake[1]. Also, only a minority of patients with eating disorders (approximately 6%) abuse diuretics[2–4]. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to an early and accurate diagnosis. -

Vitamin and Mineral Requirements in Human Nutrition
P000i-00xx 3/12/05 8:54 PM Page i Vitamin and mineral requirements in human nutrition Second edition VITPR 3/12/05 16:50 Page ii WHO Library Cataloguing-in-Publication Data Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (1998 : Bangkok, Thailand). Vitamin and mineral requirements in human nutrition : report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30 September 1998. 1.Vitamins — standards 2.Micronutrients — standards 3.Trace elements — standards 4.Deficiency diseases — diet therapy 5.Nutritional requirements I.Title. ISBN 92 4 154612 3 (LC/NLM Classification: QU 145) © World Health Organization and Food and Agriculture Organization of the United Nations 2004 All rights reserved. Publications of the World Health Organization can be obtained from Market- ing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permis- sion to reproduce or translate WHO publications — whether for sale or for noncommercial distri- bution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; e-mail: [email protected]), or to Chief, Publishing and Multimedia Service, Information Division, Food and Agriculture Organization of the United Nations, 00100 Rome, Italy. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization and the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

341 Nutrient Deficiency Or Disease Definition/Cut-Off Value
10/2019 341 Nutrient Deficiency or Disease Definition/Cut-off Value Any currently treated or untreated nutrient deficiency or disease. These include, but are not limited to, Protein Energy Malnutrition, Scurvy, Rickets, Beriberi, Hypocalcemia, Osteomalacia, Vitamin K Deficiency, Pellagra, Xerophthalmia, and Iron Deficiency. Presence of condition diagnosed, documented, or reported by a physician or someone working under a physician’s orders, or as self-reported by applicant/participant/caregiver. See Clarification for more information about self-reporting a diagnosis. Participant Category and Priority Level Category Priority Pregnant Women 1 Breastfeeding Women 1 Non-Breastfeeding Women 6 Infants 1 Children 3 Justification Nutrient deficiencies or diseases can be the result of poor nutritional intake, chronic health conditions, acute health conditions, medications, altered nutrient metabolism, or a combination of these factors, and can impact the levels of both macronutrients and micronutrients in the body. They can lead to alterations in energy metabolism, immune function, cognitive function, bone formation, and/or muscle function, as well as growth and development if the deficiency is present during fetal development and early childhood. The Centers for Disease Control and Prevention (CDC) estimates that less than 10% of the United States population has nutrient deficiencies; however, nutrient deficiencies vary by age, gender, and/or race and ethnicity (1). For certain segments of the population, nutrient deficiencies may be as high as one third of the population (1). Intake patterns of individuals can lead to nutrient inadequacy or nutrient deficiencies among the general population. Intakes of nutrients that are routinely below the Dietary Reference Intakes (DRI) can lead to a decrease in how much of the nutrient is stored in the body and how much is available for biological functions. -

Magnesium Deficiency: a Possible Cause of Thiamine Refractoriness in Wernicke-Korsakoff Encephalopathy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.37.8.959 on 1 August 1974. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1974, 37, 959-962 Magnesium deficiency: a possible cause of thiamine refractoriness in Wernicke-Korsakoff encephalopathy D. C. TRAVIESA From the Department ofNeurology, University ofMiami School of Medicine, Miami, Florida, U.S.A. SYNOPSIS The determination of blood transketolase before and serially after thiamine administra- tion, and the response of clinical symptomatology after thiamine are reported in two normo- magnesaemic patients and one hypomagnesaemic patient with acute Wernicke-Korsakoff encephalopathy. The response of the depressed blood transketolase and the clinical symptoms was retarded in the hypomagnesaemic patient. Correction of hypomagnesaemia was accompanied by the recovery of blood transketolase activity and total clearing of the ophthalmoplegia in this patient, guest. Protected by copyright. suggesting that hypomagnesaemia may be a cause of the occasional thiamine refractoriness of these patients. Previous studies have shown that the clinical vestibular nuclei (Prickett, 1934; Dreyfus and entity of Wernicke-Korsakoff encephalopathy is Victor, 1961; Dreyfus, 1965). related to an exclusive deficiency of thiamine (Phillips et al., 1952). Furthermore, improvement Blood transketolase activity is markedly re- in clinical symptoms, once the syndrome has de- duced in patients with Wernicke-Korsakoff veloped, occurs only with the repletion of thi- encephalopathy and serial assays in these patients amine. It is this obvious and seemingly pure usually reveal a rapid and essentially complete causal relationship of thiamine deficiency and recovery of the reduced blood transketolase Wernicke-Korsakoff encephalopathy that neces- activity after the parental administration of sitates the total understanding of thiamine thiamine (Brin, 1962; Dreyfus, 1962). -

Novel Mutations Associated with Inherited Human Calcium-Sensing
1 180 A García-Castaño, Novel mutations in the calcium 180:1 59–70 Clinical Study L Madariaga and others receptor gene Novel mutations associated with inherited human calcium-sensing receptor disorders: A clinical genetic study Alejandro García-Castaño1,*, Leire Madariaga1,2,*, Gustavo Pérez de Nanclares1,2, Gema Ariceta3, Sonia Gaztambide1,2 and Luis Castaño1,2 on behalf of Spanish Endocrinology Group and Renal Tube Group Correspondence should be addressed 1Biocruces Bizkaia Health Research Institute, CIBERDEM, CIBERER, Barakaldo, Spain, 2Hospital Universitario Cruces, to L Castaño UPV/EHU, Barakaldo, Spain, and 3Hospital Universitario Materno-Infantil Vall d’Hebron, Autonomous University of Email Barcelona, Barcelona, Spain LUISANTONIO. *(A García-Castaño and L Madariaga contributed equally to this work) CASTANOGONZALEZ@ osakidetza.eus Abstract Objective: Molecular diagnosis is a useful diagnostic tool in calcium metabolism disorders. The calcium-sensing receptor (CaSR) is known to play a central role in the regulation of extracellular calcium homeostasis. We performed clinical, biochemical and genetic characterization of sequence anomalies in this receptor in a cohort of 130 individuals from 82 families with suspected alterations in the CASR gene, one of the largest series described. Methods: The CASR gene was screened for mutations by polymerase chain reaction followed by direct Sanger sequencing. Results: Presumed CaSR-inactivating mutations were found in 65 patients from 26 families. These patients had hypercalcemia (median: 11.3 mg/dL) but normal or abnormally high parathyroid hormone (PTH) levels (median: 52 pg/ mL). On the other hand, presumed CaSR-activating mutations were detected in 17 patients from eight families. These patients had a median serum calcium level of 7.4 mg/dL and hypoparathyroidism (median: PTH 13 pg/mL). -
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Vitamin B12 Deficiency Can Have a Number of Possible
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vitamin B12 deficiency can have a number of possible causes. Typically it occurs in people whose digestive systems do not adequately absorb the vitamin from the foods they eat. This can be caused by: Pernicious anemia, a condition in where there is a lack of a protein called intrinsic factor. The protein, which is made in the stomach, is necessary for vitamin B12 absorption. Other causes can be gastritis, surgery in which part of the stomach and/or small intestine is removed or conditions affecting the small intestine. Vitamin B12 deficiency can also occur in pregnancy and with long- term use of acid-reducing drugs. VI.2.2 Summary of treatment benefits A deficiency of vitamin B12 (cobalamin) is manifested by fatigue, weakness, mood fluctuations, memory loss, limb weakness, difficulty walking, tingling, and paralysis. Drug treatment involves the administration of vitamin B12 by injection. If the deficiency of vitamin B12 (cobalamin) depends on the underlying disease being resolved, the vitamin supplementation continues until normal levels of vitamin B12 are achieved. If the deficiency state cannot be resolved (e.g. inadequate secretion of intrinsic factor, genetic abnormalities related to the site absorption, etc.) therapy should be continued for life. VI.2.3 Unknowns relating to treatment benefits None. VI.2.4 Summary of safety concerns Risk What is known Preventability Damage to the optic nerve Optic nerve atrophy/blindness The product should be used (atrophy)/blindness in is a risk associated with with caution. patients with Leber´s disease treatment with (a disease that affects the hydroxocobalamin in patients optic nerve and often causes with Leber´s disease. -

Mechanism of Hypokalemia in Magnesium Deficiency
SCIENCE IN RENAL MEDICINE www.jasn.org Mechanism of Hypokalemia in Magnesium Deficiency Chou-Long Huang*† and Elizabeth Kuo* *Department of Medicine, †Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center, Dallas, Texas ABSTRACT deficiency is likely associated with en- ϩ Magnesium deficiency is frequently associated with hypokalemia. Concomitant hanced renal K excretion. To support magnesium deficiency aggravates hypokalemia and renders it refractory to treat- this idea, Baehler et al.5 showed that ad- ment by potassium. Herein is reviewed literature suggesting that magnesium ministration of magnesium decreases ϩ deficiency exacerbates potassium wasting by increasing distal potassium secre- urinary K excretion and increases se- ϩ tion. A decrease in intracellular magnesium, caused by magnesium deficiency, rum K levels in a patient with Bartter releases the magnesium-mediated inhibition of ROMK channels and increases disease with combined hypomagnesemia potassium secretion. Magnesium deficiency alone, however, does not necessarily and hypokalemia. Similarly, magnesium ϩ cause hypokalemia. An increase in distal sodium delivery or elevated aldosterone replacement alone (without K ) in- ϩ levels may be required for exacerbating potassium wasting in magnesium creases serum K levels in individuals deficiency. who have hypokalemia and hypomag- nesemia and receive thiazide treatment.6 J Am Soc Nephrol 18: 2649–2652, 2007. doi: 10.1681/ASN.2007070792 Magnesium administration decreased urinary Kϩ excretion in these individuals (Dr. Charles Pak, personal communica- Hypokalemia is among the most fre- cin B, cisplatin, etc. Concomitant magne- tion, UT Southwestern Medical Center quently encountered fluid and electro- sium deficiency has long been appreci- at Dallas, July 13, 2007). -

An Infant with Chronic Hypernatremia
European Journal of Endocrinology (2006) 155 S141–S144 ISSN 0804-4643 An infant with chronic hypernatremia M L Marcovecchio Department of Paediatrics, University of Chieti, Via dei Vestini 5, 66100 Chieti, Italy (Correspondence should be addressed to M L Marcovecchio; Email: [email protected]) Abstract A 4-month-old boy was presented with failure to thrive, refusal to feed, delayed motor development, truncal hypotonia, and head lag. His plasma osmolality and sodium were significantly high, while his urine osmolality was inappropriately low and did not increase after desmopressin administration. Despite his hyperosmolality, he presented with a lack of thirst and became clearly polyuric and polydipsic only at the age of 2 years. Initial treatment with indomethacin was ineffective, while the combination of hydrochlorothiazide and amiloride was effective and well tolerated. European Journal of Endocrinology 155 S141–S144 Introduction (61 ml/kg) respectively. Other investigations including thyroid and adrenal function were normal. He was Chronic hypernatremia in young patients is generally refusing oral fluids despite being hypernatremic. the result of alterations in the mechanisms controlling A cranial magnetic resonance imaging scan excluded fluid balance (1). Excessive water loss, as in diabetes structural abnormalities in the hypophysis, hypo- insipidus, or an inadequate fluid intake, as in adipsic thalamus and surrounding area. There was no response hypernatremia, may be the underlying cause. The to 0.3 mg 1-desamino-8-D-arginine-vasopressin (DDAVP) differential diagnosis between these conditions is given intravenously (osmolality pre- 374 mosmol/kg; important in order to choose the appropriate treatment. post- 371 mosmol/kg). This suggested a tubular defect However, pitfalls in the diagnosis are often related to an causing nephrogenic diabetes insipidus (NDI). -

Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside Or Colistin Therapy: a Systematic Review
antibiotics Review Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside or Colistin Therapy: A Systematic Review Martin Scoglio 1,* , Gabriel Bronz 1, Pietro O. Rinoldi 1,2, Pietro B. Faré 3,Céline Betti 1,2, Mario G. Bianchetti 1, Giacomo D. Simonetti 1,2, Viola Gennaro 1, Samuele Renzi 4, Sebastiano A. G. Lava 5 and Gregorio P. Milani 2,6,7 1 Faculty of Biomedicine, Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] (G.B.); [email protected] (P.O.R.); [email protected] (C.B.); [email protected] (M.G.B.); [email protected] (G.D.S.); [email protected] (V.G.) 2 Department of Pediatrics, Pediatric Institute of Southern Switzerland, Ospedale San Giovanni, Ente Ospedaliero Cantonale, 6500 Bellinzona, Switzerland; [email protected] 3 Department of Internal Medicine, Ospedale La Carità, Ente Ospedaliero Cantonale, 6600 Locarno, Switzerland; [email protected] 4 Division of Hematology and Oncology, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected] 5 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1011 Lausanne, Switzerland; [email protected] 6 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy 7 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected] Citation: Scoglio, M.; Bronz, G.; Abstract: Aminoglycoside or colistin therapy may alter the renal tubular function without decreasing Rinoldi, P.O.; Faré, P.B.; Betti, C.; the glomerular filtration rate. This association has never been extensively investigated. -
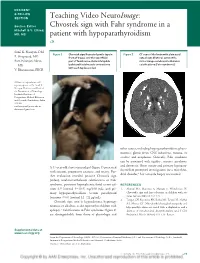
Chvostek Sign with Fahr Syndrome in a Patient with Hypoparathyroidism Sunil K
RESIDENT & FELLOW SECTION Teaching Video NeuroImage: Section Editor Chvostek sign with Fahr syndrome in a Mitchell S.V. Elkind, MD, MS patient with hypoparathyroidism Sunil K. Narayan, DM Figure 1 Chvostek sign: Repeated gentle taps in Figure 2 CT scan of the brain with plain axial P. Sivaprasad, MD front of tragus over the superficial cuts shows bilateral, symmetric, Ram Niranjan Sahoo, part of facial nerve elicits infatigable mirror-image cerebrostriothalamic MD ipsilateral facial muscle contractions calcifications (Fahr syndrome) with each tap (see video) V. Bhuvaneswari, FRCR Address correspondence and reprint requests to Dr. Sunil K. Narayan, Professor and Head of the Department of Neurology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India 605 006 [email protected] [email protected] other causes, including hyperparathyroidism; phaco- matoses; gliosis from CNS infections, trauma, or strokes; and neoplasms. Clinically, Fahr syndrome can be associated with rigidity, seizures, psychosis, and dementia. Short stature and primary hypopara- A 17-year-old short-statured girl (figure 1) presented thyroidism prompted investigations for a mitochon- with seizures, progressive cataracts, and tetany. Fur- drial disorder,2 but a muscle biopsy was normal. ther evaluation revealed positive Chvostek sign (video), cerebrostriothalamic calcifications or Fahr syndrome, persistent hypocalcemia (total serum cal- REFERENCES cium 6.5 [normal 9–10.5] mg/100 mL), and pri- 1. Ahmed MA, Martinez A, Mariam S, Whitehouse W. mary hypoparathyroidism (serum parathyroid Chvostek’s sign and hypocalcaemia in children with sei- hormone Ͻ3.0 [normal 12–72] pg/mL). zures. Seizure 2004;13:217–222. 2. Tengan CH, Kiyomoto BH, Rocha MS, Tavares VL, Gabbai Chvostek sign, seen in hypocalcemia, hypomag- AA, Moraes CT. -

Qtc Prolongation Is Associated with Hypokalemia and Hypocalcemia in Emergency Department Patients Lucy Franjic Washington University School of Medicine in St
Washington University School of Medicine Digital Commons@Becker Division of Emergency Medicine/Emergency Care Conference Abstracts and Posters Research Section 2012 QTc prolongation is associated with hypokalemia and hypocalcemia in emergency department patients Lucy Franjic Washington University School of Medicine in St. Louis Stacey House Washington University School of Medicine in St. Louis Irena Vitkovitsky Washington University School of Medicine in St. Louis S. Eliza Halcomb Washington University School of Medicine in St. Louis Follow this and additional works at: http://digitalcommons.wustl.edu/em_conf Recommended Citation Franjic, Lucy; House, Stacey L.; Vitkovitsky, Irena; Halcomb, S. Eliza, "QTc prolongation is associated with hypokalemia and hypocalcemia in emergency department patients" (2012). Conference Abstracts and Posters. Paper 12. http://digitalcommons.wustl.edu/em_conf/12 This Presentation Paper is brought to you for free and open access by the Division of Emergency Medicine/Emergency Care Research Section at Digital Commons@Becker. It has been accepted for inclusion in Conference Abstracts and Posters by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. QTc Prolongation is Associated with Hypokalemia and Hypocalcemia in Emergency Department Patients Lucy Franjic, MD Stacey L. House MD PhD, Lucy Franjic MD, Irena Vitkovitsky MD, S. Eliza Halcomb MD Washington University in St. Louis Division of Emergency Medicine Society for Academic Emergency Medicine Great Plains Regional Research Forum St. Louis, MO. September 2012 © Stacey House, 2012 QTc Prolongation Congenital Six types (LQT1-LQT6) Mutations in genes encoding potassium and sodium transmembrane channel proteins Acquired Hypokalemia, hypocalcemia, hypomagnesemia, HIV, myocardial ischemia, numerous medications and drugs (i.e. -

Hypophosphatasia: an Overview for Physicians and Medical Professionals
This publication is distributed by Soft Bones Inc., The U.S. Hypophosphatasia Foundation. Hypophosphatasia: An Overview For Physicians and Medical Professionals Michael P. Whyte, M.D. Definition of hypophosphatasia (HPP) Hypophosphatasia (HPP) is the rare genetic form of rickets or osteomalacia that features paradoxically low serum alkaline phosphatase (ALP) activity. Classification Six clinical forms represent a useful classification of HPP. 1. Perinatal Hypophosphatasia 4. Adult Hypophosphatasia 2. Infantile Hypophosphatasia 5. Odontohypophosphatasia 3. Childhood Hypophosphatasia 6. Benign Prenatal Hypophosphatasia The expressivity (disease severity) of HPP ranges greatly with the clinical consequences spanning death in utero from an essentially unmineralized skeleton to problems only with teeth during adult life. The patient’s age at which bone disease becomes apparent distinguishes the perinatal, infantile, childhood, and adult forms. Those who exhibit only dental manifestations have odonto-HPP. The benign prenatal form of HPP is the newest group and manifests skeletal deformity in utero or at birth, but in contradistinction to perinatal HPP is clearly more mild and shows significant spontaneous postnatal improvement. | 2 | Clinical Features 1. Perinatal Hypophosphatasia This is the most severe form of HPP and was almost always fatal until enzyme replacement therapy became available for HPP. At delivery, limbs are shortened and deformed and there is caput membraneceum from profound skeletal hypomineralization. Unusual osteochondral spurs may pierce the skin and protrude laterally from the midshaft of the ulnas and fibulas. There can be a high pitched cry, irritability, periodic apnea with cyanosis and bradycardia, unexplained fever, anemia, and intracranial hemorrhage. Some affected neonates live a few days, but suffer increasing respiratory compromise from defects in the thorax and hypoplastic lungs.