341 Nutrient Deficiency Or Disease Definition/Cut-Off Value
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rickets: Not a Disease of the Past LINDA S
Rickets: Not a Disease of the Past LINDA S. NIELD, M.D., West Virginia University School of Medicine, Morgantown, West Virginia PRASHANT MAHAJAN, M.D., M.P.H., Wayne State University School of Medicine, Detroit, Michigan APARNA JOSHI, M.D., Children’s Hospital of Michigan, Detroit, Michigan DEEPAK KAMAT, M.D., PH.D., Wayne State University School of Medicine, Detroit, Michigan Rickets develops when growing bones fail to mineralize. In most cases, the diagnosis is established with a thorough his- tory and physical examination and confirmed by laboratory evaluation. Nutritional rickets can be caused by inadequate intake of nutrients (vitamin D in particular); however, it is not uncommon in dark-skinned children who have limited sun exposure and in infants who are breastfed exclusively. Vitamin D–dependent rickets, type I results from abnor- malities in the gene coding for 25(OH)D3-1-a-hydroxylase, and type II results from defective vitamin D receptors. The vitamin D–resistant types are familial hypophosphatemic rickets and hereditary hypophosphatemic rickets with hyper- calciuria. Other causes of rickets include renal disease, medications, and malabsorption syndromes. Nutritional rickets is treated by replacing the deficient nutrient. Mothers who breastfeed exclusively need to be informed of the recom- mendation to give their infants vitamin D supplements beginning in the first two months of life to prevent nutritional rickets. Vitamin D–dependent rickets, type I is treated with vitamin D; management of type II is more challenging. Familial hypophosphatemic rickets is treated with phosphorus and vitamin D, whereas hereditary hypophosphatemic rickets with hypercalciuria is treated with phosphorus alone. -

Vitamin and Mineral Requirements in Human Nutrition
P000i-00xx 3/12/05 8:54 PM Page i Vitamin and mineral requirements in human nutrition Second edition VITPR 3/12/05 16:50 Page ii WHO Library Cataloguing-in-Publication Data Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (1998 : Bangkok, Thailand). Vitamin and mineral requirements in human nutrition : report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30 September 1998. 1.Vitamins — standards 2.Micronutrients — standards 3.Trace elements — standards 4.Deficiency diseases — diet therapy 5.Nutritional requirements I.Title. ISBN 92 4 154612 3 (LC/NLM Classification: QU 145) © World Health Organization and Food and Agriculture Organization of the United Nations 2004 All rights reserved. Publications of the World Health Organization can be obtained from Market- ing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permis- sion to reproduce or translate WHO publications — whether for sale or for noncommercial distri- bution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; e-mail: [email protected]), or to Chief, Publishing and Multimedia Service, Information Division, Food and Agriculture Organization of the United Nations, 00100 Rome, Italy. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization and the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Severe Malnutrition: a Global Approach. INSTITUTION International Children's Centre, Paris (France)
, DOCUMENT RESUME ED 371 865 PS 022 466 AUTHOR Pelletier, Jean-Gerard TITLE Severe Malnutrition: A Global Approach. INSTITUTION International Children's Centre, Paris (France). REPORT NO ISSN-0379-2269 PUB DATE 93 NOTE 88p. AVAILABLE FROMChildren in the Tropics, International Children's Centre, Chateau de Longchamp, Bois de Boulogne, 75016, Paris, France ($10; annual subscription: $40 for 6 issues). PUB TYPE Collected Works Serials (022) JOURNAL CIT Children in the Tropics; n208-209 1993 EDRS PRICE MF01/PC04 Plus Postage. DESCRIPTORS Biological Influences; *Child Health; Children; Cultural Influences; Developing Nations; Disease Incidence; *Diseases; Foreign Countries; Global - Approach; *Hunger; *Intervention; Medical Services; *Nutrition; Nutrition Instruction; Poverty; Program Evaluation; Rehabilitation; Socioeconomic Influences IDENTIFIERS Anthropometry; KwashisKor; Marasmus; Psychological Influences ABSTRACT This report examines the immediate and underlying causes of malnutrition in the developing world. The first section discusses the effects of malnutrition on childhood development and examines the efficacy of nutritional rehabilitation. The second section addresses the medical effects of severe malnutrition, including the onset of ponderostatural (weight) retardation, behavioral disorders, dehydration, anemia, hypothermia, hypoglycemia. and diarrhea. The third section focuses on anthropometric approaches to treating malnourished children, which treat children on an individual basis based upon their particular condition. The fourth section examines the biological effects of severe malnutrition, discussing deficiencies in serum proteins, electrolytes, trace elements, and hormonal levels, along with their immunological consequences. The fifth section explains the nutritional approach to the problem, looking at protein, vitamin, and mineral deficiencies lnd specific rehabilitation procedures and foods. The sixth section focuses on a cultural approach to malnutrition, discussing dietary and social customs that affect nutrition and eating behaviors. -

Marasmus- 1985
Postgraduate Medical Journal (1985) 61, 915-923 Postgrad Med J: first published as 10.1136/pgmj.61.720.915 on 1 October 1985. Downloaded from Marasmus- 1985 D. Barltrop and B.K. Sandhu Department ofChild Health, Charing Cross and Westminster Medical School, London, UK. Introduction Dr Wilfred J. Pearson writing for the first volume of In 1959 Jelliffe coined the term protein-calorie this Journal in 1925 defined marasmus (Greek: maras- malnutrition (PCM) of early childhood to include mos, wasting) as 'a chronic state of malnutrition of a mild, moderate and various types of severe degrees of severe grade' but pointed out that 'we must retain the malnutrition. The advent of the International System conception ofcases varying in severity from those who of Units (SI) resulted in the introduction of the term simply show an insufficient gain or stationary weight protein energy malnutrition (PEM). The World with few systemic changes, to the most extreme form Health Organisation (WHO, 1973) defined PEM as which is merely the end result of repeated nutritional follows: 'A range of pathological conditions arising or constitutional disturbances.' He described oedema from coincidental lack, in varying proportions, of in some cases as a complication of marasmus. The protein and calories, occurring most frequently in clinical picture of the syndrome, later described as infants and young children and commonly associated kwashiorkor, was not generally recognized at this with infection'. This is not dissimilar from Wilfred time, although a number of authors had described it Pearson's view of marasmus. (Czerney & Keller, 1928). In order to treat and, more importantly, to prevent a In 1933 Cicely D. -

Magnesium Deficiency: a Possible Cause of Thiamine Refractoriness in Wernicke-Korsakoff Encephalopathy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.37.8.959 on 1 August 1974. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1974, 37, 959-962 Magnesium deficiency: a possible cause of thiamine refractoriness in Wernicke-Korsakoff encephalopathy D. C. TRAVIESA From the Department ofNeurology, University ofMiami School of Medicine, Miami, Florida, U.S.A. SYNOPSIS The determination of blood transketolase before and serially after thiamine administra- tion, and the response of clinical symptomatology after thiamine are reported in two normo- magnesaemic patients and one hypomagnesaemic patient with acute Wernicke-Korsakoff encephalopathy. The response of the depressed blood transketolase and the clinical symptoms was retarded in the hypomagnesaemic patient. Correction of hypomagnesaemia was accompanied by the recovery of blood transketolase activity and total clearing of the ophthalmoplegia in this patient, guest. Protected by copyright. suggesting that hypomagnesaemia may be a cause of the occasional thiamine refractoriness of these patients. Previous studies have shown that the clinical vestibular nuclei (Prickett, 1934; Dreyfus and entity of Wernicke-Korsakoff encephalopathy is Victor, 1961; Dreyfus, 1965). related to an exclusive deficiency of thiamine (Phillips et al., 1952). Furthermore, improvement Blood transketolase activity is markedly re- in clinical symptoms, once the syndrome has de- duced in patients with Wernicke-Korsakoff veloped, occurs only with the repletion of thi- encephalopathy and serial assays in these patients amine. It is this obvious and seemingly pure usually reveal a rapid and essentially complete causal relationship of thiamine deficiency and recovery of the reduced blood transketolase Wernicke-Korsakoff encephalopathy that neces- activity after the parental administration of sitates the total understanding of thiamine thiamine (Brin, 1962; Dreyfus, 1962). -

Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
medical sciences Review Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia Faheemuddin Ahmed 1,* and Abdul Mohammed 2 1 OSF Saint Anthony Medical Center, 5666 E State St, Rockford, IL 61108, USA 2 Advocate Illinois Masonic Medical Center, 833 W Wellington Ave, Chicago, IL 60657, USA; [email protected] * Correspondence: [email protected] Received: 20 February 2019; Accepted: 2 April 2019; Published: 4 April 2019 Abstract: Magnesium is the fourth most abundant cation in the body and the second most abundant intracellular cation. It plays an important role in different organ systems at the cellular and enzymatic levels. Despite its importance, it still has not received the needed attention either in the medical literature or in clinical practice in comparison to other electrolytes like sodium, potassium, and calcium. Hypomagnesemia can lead to many clinical manifestations with some being life-threatening. The reported incidence is less likely than expected in the general population. We present a comprehensive review of different aspects of magnesium physiology and hypomagnesemia which can help clinicians in understanding, identifying, and treating this disorder. Keywords: magnesium; proton pump inhibitors; diuretics; hypomagnesemia 1. Introduction Magnesium is one of the most abundant cation in the body as well as an abundant intracellular cation. It plays an important role in molecular, biochemical, physiological, and pharmacological functions in the body. The importance of magnesium is well known, but still it is the forgotten electrolyte. The reason for it not getting the needed attention is because of rare symptomatology until levels are really low and also because of a lack of proper understanding of magnesium physiology. -

VITAMIN DEFICIENCIES in RELATION to the EYE* by MEKKI EL SHEIKH Sudan VITAMINS Are Essential Constituents Present in Minute Amounts in Natural Foods
Br J Ophthalmol: first published as 10.1136/bjo.44.7.406 on 1 July 1960. Downloaded from Brit. J. Ophthal. (1960) 44, 406. VITAMIN DEFICIENCIES IN RELATION TO THE EYE* BY MEKKI EL SHEIKH Sudan VITAMINS are essential constituents present in minute amounts in natural foods. If these constituents are removed or deficient such foods are unable to support nutrition and symptoms of deficiency or actual disease develop. Although vitamins are unconnected with energy and protein supplies, yet they are necessary for complete normal metabolism. They are, however, not invariably present in the diet under all circumstances. Childhood and periods of growth, heavy work, childbirth, and lactation all demand the supply of more vitamins, and under these conditions signs of deficiency may be present, although the average intake is not altered. The criteria for the fairly accurate diagnosis of vitamin deficiencies are evidence of deficiency, presence of signs and symptoms, and improvement on supplying the deficient vitamin. It is easy to discover signs and symptoms of vitamin deficiencies, but it is not easy to tell that this or that vitamin is actually deficient in the diet of a certain individual, unless one is able to assess the exact daily intake of food, a process which is neither simple nor practical. Another way of approach is the assessment of the vitamin content in the blood or the measurement of the course of dark adaptation which demon- strates a real deficiency of vitamin A (Adler, 1953). Both tests entail more or less tedious laboratory work which is not easy in a provincial hospital. -

Pathogenesis and Diagnostic Criteria for Rickets and Osteomalacia
Endocrine Journal 2015, 62 (8), 665-671 OPINION Pathogenesis and diagnostic criteria for rickets and osteomalacia —Proposal by an expert panel supported by Ministry of Health, Labour and Welfare, Japan, The Japanese Society for Bone and Mineral Research and The Japan Endocrine Society Seiji Fukumoto1), Keiichi Ozono2), Toshimi Michigami3), Masanori Minagawa4), Ryo Okazaki5), Toshitsugu Sugimoto6), Yasuhiro Takeuchi7) and Toshio Matsumoto1) 1)Fujii Memorial Institute of Medical Sciences, Tokushima University, Tokushima 770-8503, Japan 2)Department of Pediatrics, Osaka University Graduate School of Medicine, Suita 565-0871, Japan 3)Department of Bone and Mineral Research, Research Institute, Osaka Medical Center for Maternal and Child Health, Izumi 594-1101, Japan 4)Department of Endocrinology, Chiba Children’s Hospital, Chiba 266-0007, Japan 5)Third Department of Medicine, Teikyo University Chiba Medical Center, Ichihara 299-0111, Japan 6)Internal Medicine 1, Shimane University Faculty of Medicine, Izumo 693-8501, Japan 7)Division of Endocrinology, Toranomon Hospital Endocrine Center, Tokyo 105-8470, Japan Abstract. Rickets and osteomalacia are diseases characterized by impaired mineralization of bone matrix. Recent investigations revealed that the causes for rickets and osteomalacia are quite variable. While these diseases can severely impair the quality of life of the affected patients, rickets and osteomalacia can be completely cured or at least respond to treatment when properly diagnosed and treated according to the specific causes. On the other hand, there are no standard criteria to diagnose rickets or osteomalacia nationally and internationally. Therefore, we summarize the definition and pathogenesis of rickets and osteomalacia, and propose the diagnostic criteria and a flowchart for the differential diagnosis of various causes for these diseases. -
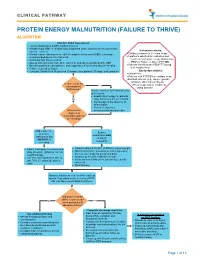
Protein Energy Malnutrition (Failure to Thrive) Algorithm
CLINICAL PATHWAY PROTEIN ENERGY MALNUTRITION (FAILURE TO THRIVE) ALGORITHM Conduct Initial Assessment • History and physical (H&P), nutrition focused • Weight height, BMI, % of ideal body weight and exam: assess severity (symmetric edema = severe) Inclusion criteria: • • Consider basic labs based on H&P; A complete blood count (CBC) is strongly Children newborn to 21 years of age • recommended due to risk of anemia Inpatients admitted for evaluation and • Additional labs based on H&P treatment of Protein Energy Malnutrition • Assess micronutrients: iron, zinc, vitamin D, and others as indicated by H&P (PEM) or Failure to thrive (FTT) OR • • Baseline potassium, phosphorus, and magnesium if concerned about re-feeding Patients identified with PEM/FTT during • Calorie count up to 3 days their hospital stay. • Consults: Social Work, Registered Dietician, Occupational Therapy, and Lactation Exclusion criteria: • Outpatients • Patients with FTT/PEM secondary to an identified concern (e.g., cancer, genetic condition, other chronic illness). Is there a risk for •Pts w/ suspected or confirmed micronutrient Yes eating disorder deficiencies? Initiate treatment for micronutrients deficiencies: • Empiric zinc therapy for patients No older than 6 months for 1 month • Iron therapy in the absence of inflammation • Vitamin D and other What are the micronutrients based on labs degrees of malnutrition and risk of refeeding? Mild, moderate, Severe or severe malnutrition AND malnutrition but at risk of NO RISK of refeeding refeeding • • Initiate feeding per recommended Initiate feeding at 30-50% of RDA for current weight • daily allowance (RDA) for current Monitor potassium, phosphorus, and magnesium weight and age once to twice a day for a total of 4 days • • Use PO route if patient is able to Advance by 10-20% if labs are normal • take 70% of estimated calories If labs abnormal hold off on advancing feed until orally corrected • Start thiamine Advance calories to meet level for catch up growth. -

Mechanism of Hypokalemia in Magnesium Deficiency
SCIENCE IN RENAL MEDICINE www.jasn.org Mechanism of Hypokalemia in Magnesium Deficiency Chou-Long Huang*† and Elizabeth Kuo* *Department of Medicine, †Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center, Dallas, Texas ABSTRACT deficiency is likely associated with en- ϩ Magnesium deficiency is frequently associated with hypokalemia. Concomitant hanced renal K excretion. To support magnesium deficiency aggravates hypokalemia and renders it refractory to treat- this idea, Baehler et al.5 showed that ad- ment by potassium. Herein is reviewed literature suggesting that magnesium ministration of magnesium decreases ϩ deficiency exacerbates potassium wasting by increasing distal potassium secre- urinary K excretion and increases se- ϩ tion. A decrease in intracellular magnesium, caused by magnesium deficiency, rum K levels in a patient with Bartter releases the magnesium-mediated inhibition of ROMK channels and increases disease with combined hypomagnesemia potassium secretion. Magnesium deficiency alone, however, does not necessarily and hypokalemia. Similarly, magnesium ϩ cause hypokalemia. An increase in distal sodium delivery or elevated aldosterone replacement alone (without K ) in- ϩ levels may be required for exacerbating potassium wasting in magnesium creases serum K levels in individuals deficiency. who have hypokalemia and hypomag- nesemia and receive thiazide treatment.6 J Am Soc Nephrol 18: 2649–2652, 2007. doi: 10.1681/ASN.2007070792 Magnesium administration decreased urinary Kϩ excretion in these individuals (Dr. Charles Pak, personal communica- Hypokalemia is among the most fre- cin B, cisplatin, etc. Concomitant magne- tion, UT Southwestern Medical Center quently encountered fluid and electro- sium deficiency has long been appreci- at Dallas, July 13, 2007). -

Nutritional Dermatoses in the Hospitalized Patient
HOSPITAL CONSULT IN PARTNERSHIP WITH THE SOCIETY FOR DERMATOLOGY HOSPITALISTS Nutritional Dermatoses in the Hospitalized Patient Melissa Hoffman, MS; Robert G. Micheletti, MD; Bridget E. Shields, MD Nutritional deficiencies may arise from inadequate nutrient intake, abnormal nutrient absorption, or improper nutrient PRACTICE POINTS utilization.4 Unfortunately, no standardized algorithm for • Nutritional deficiencies are common in hospitalized screening and diagnosing patients with malnutrition exists, patients and often go unrecognized. making early physical examination findings of utmost • Awareness of the risk factors predisposing patients importance. Herein, we present a review of acquired nutri- to nutritional deficiencies and the cutaneous manifes- tional deficiency dermatoses in the inpatient setting. tations associated with undernutrition can promote copy early diagnosis. Protein-Energy Malnutrition • When investigating cutaneous findings, undernutri- tion should be considered in patients with chronic Protein-energy malnutrition (PEM) refers to a set of infections, malabsorptive states, psychiatric illness, related disorders that include marasmus, kwashiorkor and strict dietary practices, as well as in those using (KW), and marasmic KW. These conditions frequently are certain medications. seen in developing countries but also have been reported 5 • Prompt nutritional supplementation can prevent patient in developed nations. Marasmus occurs from a chronic morbidity and mortality and reverse skin disease. deficiencynot of protein and calories. Decreased insulin pro- duction and unopposed catabolism result in sarcopenia and loss of bone and subcutaneous fat.6 Affected patients include children who are less than 60% ideal body weight Cutaneous disease may be the first manifestation of an underlying nutri- 7 tional deficiency, highlighting the importance of early recognition by der- (IBW) without edema or hypoproteinemia. -

INCIDENCE of UTRITIONAL DEFICIENCY DISEASES AMONG the BANTU and COLOURED Populanons in SOUTH AFRICA AS REFLECTED by the RESULTS of a QUESTIONNAIRE SURVEY*
504 S.A. MEDICAL JOUR AL 18 June 1966 N22 (Supplemenr-SlJIl1h African Jo//mal of Nurririun) INCIDENCE OF UTRITIONAL DEFICIENCY DISEASES AMONG THE BANTU AND COLOURED POPULAnONS IN SOUTH AFRICA AS REFLECTED BY THE RESULTS OF A QUESTIONNAIRE SURVEY* J. F. POTGIETER,** S. A. FELLINGHAM.t AND M. L. ESER** Although it is today generally conceded by nutritionists (vii) Crireria for rhe clillical diagllosis of the specific IIl11ri that malnutrition is a major problem in the Republic and rional diseases, and assumes serious proportions in the younger non-White (viii). Descripriolls of 1I01l-specific sigm alld symprol1ls. In formatIon under (vii) and (viii) was provided in order t age-groups, no reliable information is at present available facilitate the diagnosis of the various diseases and the recoo which gives a true picture of the extent of the problem nition of signs and symptoms referred to in the questionnair~. throughout the country. This information was considered necessary as, without it, the cnteria adopted by different practitioners and their evaluatior To obtain detailed and accurate information on the of the severity of the diseases might vary considerably. prevalence of nutritional disease in all areas of the coun In an accompanying letter, the doctors were asked to keer try, it would be necessary to conduct field surveys on records for a 4-week period during May/June and anotheI samples from all sections of the population-a large and during NovemberfDecember 1960. An additional copy of the unwieldy task. As it was thought that medical practitioners questionnaire was included for use during the latter period.