Hypokalaemia in a Woman with Eating Disorder
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE IMPORTANCE of NUTRITION AS the BEST MEDICINE for EATING DISORDERS Carolyn Coker Ross, MD, MPH
DIET AND NUTRITION THE IMPORTANCE OF NUTRITION AS THE BEST MEDICINE FOR EATING DISORDERS Carolyn Coker Ross, MD, MPH ver seven million girls and women groups. Current research demonstrates to 24, and the suicide rate was 75 times and one million boys and men that eating disorder symptoms may be as higher. will suffer from an eating disorder common or more common among certain Medical consequences of eating disor- in their lifetime. Up to 3.7% of ethnic groups (Asians, blacks, and Hispan- ders include arrested sexual maturity and O 6 females will be diagnosed with anorexia ics) when compared with whites. There growth failure in prepubertal patients. nervosa and an estimated 4.2% will have was no difference found in dieting and Many with eating disorders may look and bulimia nervosa.1 The majority of adoles- restraint scores between Asian, Latino, feel deceptively well and may have normal cent patients seen in referral centers fit and white adolescent girls and boys7 and electrograms but are still at high risk for into a third category, “eating disorder not no difference in binging or BED in obese cardiac arrhythmias and sudden death. otherwise specified” or EDNOS and do patients who sought to lose weight with Prolonged amenorrhea is associated with not fit strict criteria for either anorexia or bariatric surgery.8 These changes may be an increased risk of osteopenia and rate of bulimia.2 Nineteen percent of college- related to an extension of cultural ideals in fractures. Neuroimaging studies with com- aged females are bulimic; many go undi- these ethnic populations of what is attrac- puterized tomography (CT) have demon- agnosed until much later. -

Common Signs and Symptoms of Eating Disorders (Anorexia/Bulimia)
Common Signs and Symptoms of Eating Disorders (Anorexia/Bulimia) 1. Dramatic weight loss in a relatively short period of time. 2. Wearing big or baggy clothes or dressing in layers to hide body and/or weight loss. 3. Obsession with calories and fat content of foods. 4. Obsession with continuous exercise. 5. Frequent trips to the bathroom immediately following meals (sometimes accompanied with water running in the bathroom for a long period of time to hide the sound of vomiting). 6. Visible food restriction and self-starvation. 7. Visible bingeing and/or purging. 8. Use or hiding use of diet pills, laxatives, ipecac syrup (can cause immediate death!) or enemas. 9. Isolation. Fear of eating around and with others. 10. Hiding food in strange places (closets, cabinets, suitcases, under the bed) to avoid eating (Anorexia) or to eat at a later time (Bulimia). 11. Flushing uneaten food down the toilet (can cause sewage problems). 12. Vague or secretive eating patterns. 13. Keeping a "food diary" or lists that consists of food and/or behaviors (ie., purging, restricting, calories consumed, exercise, etc.) 14. Pre-occupation or obsession with food, weight (even if “average” weight or thin), and/or cooking. 15. Visiting websites that promote unhealthy ways to lose weight. 16. Reading books about weight loss and eating disorders. 17. Unusual food rituals: shifting the food around on the plate to look eaten; cutting food into tiny pieces; making sure the fork avoids contact with the lips (using teeth to scrap food off the fork or spoon); chewing food and spitting it out, but not swallowing; dropping food into napkin on lap to later throw away. -

Section 15: Treatment of Eating Disorders
Formulary and Prescribing Guidelines SECTION 15: TREATMENT OF EATING DISORDERS Section 15. Treatment of eating disorders 15.1 Introduction Please review the Trust document “Guidelines for the assessment and treatment of eating disorders” in the CAMHS Operational Policy. When screening for eating disorders one or two simple questions should be considered for use with specific target groups 1. Do you think you have an eating problem? 2. Do you worry excessively about your weight?’ Early detection may be helped by five screening questions using The SCOFF questionnaire. A score of two or more positive answers should raise clinical suspicion and lead to an in depth diagnostic evaluation. 1. Do you ever make yourself Sick because you feel uncomfortably full? 2. Do you worry you have lost Control over how much you eat? 3. Have you recently lost more than One stone in a three month period? 4. Do you believe yourself to be Fat when others say you are too thin? 5. Would you say that Food dominates your life? It is important to take into account that clients with eating disorders can develop Acute Kidney Injury through a variety of mechanisms associated with each condition. Clinicians should be vigilant in the monitoring of physical health especially serum creatinine and levels of hydration.3 15.2 Anorexia nervosa The following would represent a reasonable initial screen for Anorexia Nervosa in primary care if there are no other indications or diagnostic concerns: Full Blood Count, ESR, Urea and Electrolytes, Creatinine, Liver Function Tests, Random Blood Glucose, Urinalysis, ECG (should be considered in all cases and essential if symptoms/signs of compromised cardiac function, bradycardia, electrolyte abnormality and/or BMI less than 15 kg/m2 or equivalent on centile chart). -

Anorexia/Cachexia Heart Failure Symptom Management Guideline for Adults, Age 19 and Older in British Columbia
Anorexia/Cachexia Heart Failure Symptom Management Guideline For adults, age 19 and older in British Columbia What is anorexia? Anorexia is a syndrome characterized by some or all of the following symptoms: loss of appetite, nausea, early satiety, weakness, fatigue, food aversion, and significant physical and/or psychological symptoms. Causes of anorexia are multifactorial and include fatigue, dyspnea, medication side-effects, nausea, depression, anxiety and sodium restricted diets, which may all be found in patients with heart failure. What is cachexia? Cachexia is a syndrome characterized by severe body weight, fat and muscle loss and increased protein catabolism due to underlying disease. The prevalence of cachexia is 16–42% in the heart failure population and is associated with a 50%, 18 month mortality risk independent of variables such as ejection fraction, age and functional ability. How is cachexia diagnosed? Chronic condition with >5% weight loss in <12 months; or body mass index (BMI) <20kg/m2; and 3 out of 5 additional criteria: 1) Fatigue, 2) Decreased muscle strength, 3) Anorexia, 4) Low muscle mass, 5) Abnormal biochemistry *Blood testing to diagnose cachexia in advanced stages of disease is not advocated. Reminder: Malnutrition also affects prognosis in patients with heart failure and is often found in early transitions of the disease. However this symptom management guideline will focus on the assessment and treatment of anorexia and cachexia. Approach to Managing Anorexia/Cachexia Assessment History: When did weight loss begin? How much weight was lost? Obtain baseline (dry) weight. How is [the patients] appetite? What do they eat or drink on a typical day? How has weight loss affected mood? Ask about: nausea, early satiety, dyspnea, poor oral hygiene, dysphagia, malabsorption, bowel habits. -

Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
medical sciences Review Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia Faheemuddin Ahmed 1,* and Abdul Mohammed 2 1 OSF Saint Anthony Medical Center, 5666 E State St, Rockford, IL 61108, USA 2 Advocate Illinois Masonic Medical Center, 833 W Wellington Ave, Chicago, IL 60657, USA; [email protected] * Correspondence: [email protected] Received: 20 February 2019; Accepted: 2 April 2019; Published: 4 April 2019 Abstract: Magnesium is the fourth most abundant cation in the body and the second most abundant intracellular cation. It plays an important role in different organ systems at the cellular and enzymatic levels. Despite its importance, it still has not received the needed attention either in the medical literature or in clinical practice in comparison to other electrolytes like sodium, potassium, and calcium. Hypomagnesemia can lead to many clinical manifestations with some being life-threatening. The reported incidence is less likely than expected in the general population. We present a comprehensive review of different aspects of magnesium physiology and hypomagnesemia which can help clinicians in understanding, identifying, and treating this disorder. Keywords: magnesium; proton pump inhibitors; diuretics; hypomagnesemia 1. Introduction Magnesium is one of the most abundant cation in the body as well as an abundant intracellular cation. It plays an important role in molecular, biochemical, physiological, and pharmacological functions in the body. The importance of magnesium is well known, but still it is the forgotten electrolyte. The reason for it not getting the needed attention is because of rare symptomatology until levels are really low and also because of a lack of proper understanding of magnesium physiology. -
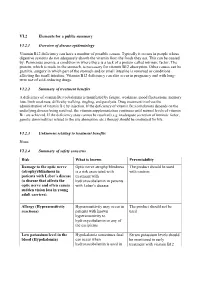
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Vitamin B12 Deficiency Can Have a Number of Possible
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vitamin B12 deficiency can have a number of possible causes. Typically it occurs in people whose digestive systems do not adequately absorb the vitamin from the foods they eat. This can be caused by: Pernicious anemia, a condition in where there is a lack of a protein called intrinsic factor. The protein, which is made in the stomach, is necessary for vitamin B12 absorption. Other causes can be gastritis, surgery in which part of the stomach and/or small intestine is removed or conditions affecting the small intestine. Vitamin B12 deficiency can also occur in pregnancy and with long- term use of acid-reducing drugs. VI.2.2 Summary of treatment benefits A deficiency of vitamin B12 (cobalamin) is manifested by fatigue, weakness, mood fluctuations, memory loss, limb weakness, difficulty walking, tingling, and paralysis. Drug treatment involves the administration of vitamin B12 by injection. If the deficiency of vitamin B12 (cobalamin) depends on the underlying disease being resolved, the vitamin supplementation continues until normal levels of vitamin B12 are achieved. If the deficiency state cannot be resolved (e.g. inadequate secretion of intrinsic factor, genetic abnormalities related to the site absorption, etc.) therapy should be continued for life. VI.2.3 Unknowns relating to treatment benefits None. VI.2.4 Summary of safety concerns Risk What is known Preventability Damage to the optic nerve Optic nerve atrophy/blindness The product should be used (atrophy)/blindness in is a risk associated with with caution. patients with Leber´s disease treatment with (a disease that affects the hydroxocobalamin in patients optic nerve and often causes with Leber´s disease. -

Dsm-5 Diagnostic Criteria for Eating Disorders Anorexia Nervosa
DSM-5 DIAGNOSTIC CRITERIA FOR EATING DISORDERS ANOREXIA NERVOSA DIAGNOSTIC CRITERIA To be diagnosed with anorexia nervosa according to the DSM-5, the following criteria must be met: 1. Restriction of energy intaKe relative to requirements leading to a significantly low body weight in the context of age, sex, developmental trajectory, and physical health. 2. Intense fear of gaining weight or becoming fat, even though underweight. 3. Disturbance in the way in which one's body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or denial of the seriousness of the current low body weight. Even if all the DSM-5 criteria for anorexia are not met, a serious eating disorder can still be present. Atypical anorexia includes those individuals who meet the criteria for anorexia but who are not underweight despite significant weight loss. Research studies have not found a difference in the medical and psychological impacts of anorexia and atypical anorexia. BULIMIA NERVOSA DIAGNOSTIC CRITERIA According to the DSM-5, the official diagnostic criteria for bulimia nervosa are: • Recurrent episodes of binge eating. An episode of binge eating is characterized by both of the following: o Eating, in a discrete period of time (e.g. within any 2-hour period), an amount of food that is definitely larger than most people would eat during a similar period of time and under similar circumstances. o A sense of lacK of control over eating during the episode (e.g. a feeling that one cannot stop eating or control what or how much one is eating). -

Cancer Cachexia and Fatigue
CME Palliative care Cancer cachexia and mechanisms (Fig 1). The cachectic Other cachectic factors patient is analogous to an accelerating Cachexia can occur in the absence of car running out of petrol. The anorexia anorexia, suggesting that catabolic fatigue component of cancer cachexia reduces mediators produced by tumour or host fuel supply (by ca 300–500 kcal/day) cells are involved in the cancer cachexia whilst accelerated metabolic cycling Grant D Stewart BSc(Hons) MBChB MRCS(Ed), process.9 Experimental cachexia models drives hypermetabolism (by ca Surgical Research Fellow suggest pro-inflammatory cytokines, 100–200 kcal/day). There are also the Richard JE Skipworth BSc(Hons) MBChB such as tumour necrosis factor- , inter- direct catabolic effects of muscle proteol- α MRCS(Ed), Surgical Research Fellow leukin (IL)-6, IL-1 and interferon- , can ysis and lipolysis. These changes underlie γ Kenneth CH Fearon MBChB(Hons) MD all play a role. Activation of the neuro- a key paradox of cachexia: whilst meta- FRCS(Glas) FRCS(Ed) FRCS(Eng), Professor of endocrine stress response is also thought bolic rate may be increased, overall (or Surgical Oncology to be important. Potential mediators total) energy expenditure is decreased Department of Clinical and Surgical Sciences include increased adrenergic activity, ele- due to a fall in physical activity.7 (Surgery), University of Edinburgh, Royal vated cortisol, low insulin and increased Infirmary, Edinburgh activity of the renin-angiotensin system.1 Anorexia With regard to tumour-specific Clin Med 2006;6:140–3 The anorexia component of cancer cachectic factors, proteolysis-inducing cachexia has both a neurohumoral mech- factor (PIF) is produced by tumours and anism due to disturbance of the central excreted in the urine of patients with Background physiological mechanisms controlling cancer cachexia. -

Mechanism of Hypokalemia in Magnesium Deficiency
SCIENCE IN RENAL MEDICINE www.jasn.org Mechanism of Hypokalemia in Magnesium Deficiency Chou-Long Huang*† and Elizabeth Kuo* *Department of Medicine, †Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center, Dallas, Texas ABSTRACT deficiency is likely associated with en- ϩ Magnesium deficiency is frequently associated with hypokalemia. Concomitant hanced renal K excretion. To support magnesium deficiency aggravates hypokalemia and renders it refractory to treat- this idea, Baehler et al.5 showed that ad- ment by potassium. Herein is reviewed literature suggesting that magnesium ministration of magnesium decreases ϩ deficiency exacerbates potassium wasting by increasing distal potassium secre- urinary K excretion and increases se- ϩ tion. A decrease in intracellular magnesium, caused by magnesium deficiency, rum K levels in a patient with Bartter releases the magnesium-mediated inhibition of ROMK channels and increases disease with combined hypomagnesemia potassium secretion. Magnesium deficiency alone, however, does not necessarily and hypokalemia. Similarly, magnesium ϩ cause hypokalemia. An increase in distal sodium delivery or elevated aldosterone replacement alone (without K ) in- ϩ levels may be required for exacerbating potassium wasting in magnesium creases serum K levels in individuals deficiency. who have hypokalemia and hypomag- nesemia and receive thiazide treatment.6 J Am Soc Nephrol 18: 2649–2652, 2007. doi: 10.1681/ASN.2007070792 Magnesium administration decreased urinary Kϩ excretion in these individuals (Dr. Charles Pak, personal communica- Hypokalemia is among the most fre- cin B, cisplatin, etc. Concomitant magne- tion, UT Southwestern Medical Center quently encountered fluid and electro- sium deficiency has long been appreci- at Dallas, July 13, 2007). -

An Infant with Chronic Hypernatremia
European Journal of Endocrinology (2006) 155 S141–S144 ISSN 0804-4643 An infant with chronic hypernatremia M L Marcovecchio Department of Paediatrics, University of Chieti, Via dei Vestini 5, 66100 Chieti, Italy (Correspondence should be addressed to M L Marcovecchio; Email: [email protected]) Abstract A 4-month-old boy was presented with failure to thrive, refusal to feed, delayed motor development, truncal hypotonia, and head lag. His plasma osmolality and sodium were significantly high, while his urine osmolality was inappropriately low and did not increase after desmopressin administration. Despite his hyperosmolality, he presented with a lack of thirst and became clearly polyuric and polydipsic only at the age of 2 years. Initial treatment with indomethacin was ineffective, while the combination of hydrochlorothiazide and amiloride was effective and well tolerated. European Journal of Endocrinology 155 S141–S144 Introduction (61 ml/kg) respectively. Other investigations including thyroid and adrenal function were normal. He was Chronic hypernatremia in young patients is generally refusing oral fluids despite being hypernatremic. the result of alterations in the mechanisms controlling A cranial magnetic resonance imaging scan excluded fluid balance (1). Excessive water loss, as in diabetes structural abnormalities in the hypophysis, hypo- insipidus, or an inadequate fluid intake, as in adipsic thalamus and surrounding area. There was no response hypernatremia, may be the underlying cause. The to 0.3 mg 1-desamino-8-D-arginine-vasopressin (DDAVP) differential diagnosis between these conditions is given intravenously (osmolality pre- 374 mosmol/kg; important in order to choose the appropriate treatment. post- 371 mosmol/kg). This suggested a tubular defect However, pitfalls in the diagnosis are often related to an causing nephrogenic diabetes insipidus (NDI). -

Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside Or Colistin Therapy: a Systematic Review
antibiotics Review Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside or Colistin Therapy: A Systematic Review Martin Scoglio 1,* , Gabriel Bronz 1, Pietro O. Rinoldi 1,2, Pietro B. Faré 3,Céline Betti 1,2, Mario G. Bianchetti 1, Giacomo D. Simonetti 1,2, Viola Gennaro 1, Samuele Renzi 4, Sebastiano A. G. Lava 5 and Gregorio P. Milani 2,6,7 1 Faculty of Biomedicine, Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] (G.B.); [email protected] (P.O.R.); [email protected] (C.B.); [email protected] (M.G.B.); [email protected] (G.D.S.); [email protected] (V.G.) 2 Department of Pediatrics, Pediatric Institute of Southern Switzerland, Ospedale San Giovanni, Ente Ospedaliero Cantonale, 6500 Bellinzona, Switzerland; [email protected] 3 Department of Internal Medicine, Ospedale La Carità, Ente Ospedaliero Cantonale, 6600 Locarno, Switzerland; [email protected] 4 Division of Hematology and Oncology, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected] 5 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1011 Lausanne, Switzerland; [email protected] 6 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy 7 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected] Citation: Scoglio, M.; Bronz, G.; Abstract: Aminoglycoside or colistin therapy may alter the renal tubular function without decreasing Rinoldi, P.O.; Faré, P.B.; Betti, C.; the glomerular filtration rate. This association has never been extensively investigated. -

Cancer Anorexia Cachexia Syndrome (CACS)
Cancer Anorexia Cachexia Syndrome (CACS) Amanda Werner, RN, BSN Supportive Care and Vitality Clinics Moffitt Cancer Center Objectives Define CACS Identify contributing factors in CACS Describe the effects of CACS on patient outcomes 58 year old Male Lung Cancer No appetite Significant weight loss: muscle and fat Decreased physical ability and function Family forcing food Patient socially withdrawn Cancer Anorexia Cachexia Syndrome (CACS) Multifactoral syndrome Negative protein and energy balance Ongoing loss of skeletal muscle mass (with/without loss of fat mass) Leads to progressive functional impairment Underlying Mechanisms Symptoms of CACS Poor appetite Involuntary weight loss Increased fatigue Loss of physical strength Cachexia is NOT… Starvation Fully reversed by conventional nutritional support or artificial nutrition Intentional Negative Outcomes Treatment ◦ Poor tolerance to treatment options ◦ Not eligible for treatment due to performance status Physical ◦ Decreased function and ability to complete ADLs Psychosocial ◦ Decreased quality of life ◦ Altered body image ◦ Source of patient/family emotional distress and conflict PATIENTS & CAREGIVERS NEED SUPPORT TO COPE WITH THE DISTRESS OF CACHEXIA 10 Prevalence Varies by tumor type Under recognized ◦ ½ of cancer patients have cachexia ◦ Approx. 30% die from cachexia Under treated ◦ Condition could be present in an obese patient CACS Nutritional Impact Primary Cachexia CACS direct impact on nutrition Secondary Cachexia Impact of cancer & treatment Tertiary