Novel Mutations Associated with Inherited Human Calcium-Sensing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
medical sciences Review Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia Faheemuddin Ahmed 1,* and Abdul Mohammed 2 1 OSF Saint Anthony Medical Center, 5666 E State St, Rockford, IL 61108, USA 2 Advocate Illinois Masonic Medical Center, 833 W Wellington Ave, Chicago, IL 60657, USA; [email protected] * Correspondence: [email protected] Received: 20 February 2019; Accepted: 2 April 2019; Published: 4 April 2019 Abstract: Magnesium is the fourth most abundant cation in the body and the second most abundant intracellular cation. It plays an important role in different organ systems at the cellular and enzymatic levels. Despite its importance, it still has not received the needed attention either in the medical literature or in clinical practice in comparison to other electrolytes like sodium, potassium, and calcium. Hypomagnesemia can lead to many clinical manifestations with some being life-threatening. The reported incidence is less likely than expected in the general population. We present a comprehensive review of different aspects of magnesium physiology and hypomagnesemia which can help clinicians in understanding, identifying, and treating this disorder. Keywords: magnesium; proton pump inhibitors; diuretics; hypomagnesemia 1. Introduction Magnesium is one of the most abundant cation in the body as well as an abundant intracellular cation. It plays an important role in molecular, biochemical, physiological, and pharmacological functions in the body. The importance of magnesium is well known, but still it is the forgotten electrolyte. The reason for it not getting the needed attention is because of rare symptomatology until levels are really low and also because of a lack of proper understanding of magnesium physiology. -

Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside Or Colistin Therapy: a Systematic Review
antibiotics Review Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside or Colistin Therapy: A Systematic Review Martin Scoglio 1,* , Gabriel Bronz 1, Pietro O. Rinoldi 1,2, Pietro B. Faré 3,Céline Betti 1,2, Mario G. Bianchetti 1, Giacomo D. Simonetti 1,2, Viola Gennaro 1, Samuele Renzi 4, Sebastiano A. G. Lava 5 and Gregorio P. Milani 2,6,7 1 Faculty of Biomedicine, Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] (G.B.); [email protected] (P.O.R.); [email protected] (C.B.); [email protected] (M.G.B.); [email protected] (G.D.S.); [email protected] (V.G.) 2 Department of Pediatrics, Pediatric Institute of Southern Switzerland, Ospedale San Giovanni, Ente Ospedaliero Cantonale, 6500 Bellinzona, Switzerland; [email protected] 3 Department of Internal Medicine, Ospedale La Carità, Ente Ospedaliero Cantonale, 6600 Locarno, Switzerland; [email protected] 4 Division of Hematology and Oncology, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected] 5 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1011 Lausanne, Switzerland; [email protected] 6 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy 7 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected] Citation: Scoglio, M.; Bronz, G.; Abstract: Aminoglycoside or colistin therapy may alter the renal tubular function without decreasing Rinoldi, P.O.; Faré, P.B.; Betti, C.; the glomerular filtration rate. This association has never been extensively investigated. -
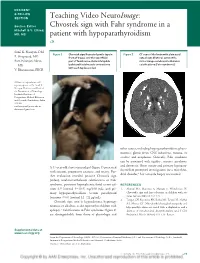
Chvostek Sign with Fahr Syndrome in a Patient with Hypoparathyroidism Sunil K
RESIDENT & FELLOW SECTION Teaching Video NeuroImage: Section Editor Chvostek sign with Fahr syndrome in a Mitchell S.V. Elkind, MD, MS patient with hypoparathyroidism Sunil K. Narayan, DM Figure 1 Chvostek sign: Repeated gentle taps in Figure 2 CT scan of the brain with plain axial P. Sivaprasad, MD front of tragus over the superficial cuts shows bilateral, symmetric, Ram Niranjan Sahoo, part of facial nerve elicits infatigable mirror-image cerebrostriothalamic MD ipsilateral facial muscle contractions calcifications (Fahr syndrome) with each tap (see video) V. Bhuvaneswari, FRCR Address correspondence and reprint requests to Dr. Sunil K. Narayan, Professor and Head of the Department of Neurology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India 605 006 [email protected] [email protected] other causes, including hyperparathyroidism; phaco- matoses; gliosis from CNS infections, trauma, or strokes; and neoplasms. Clinically, Fahr syndrome can be associated with rigidity, seizures, psychosis, and dementia. Short stature and primary hypopara- A 17-year-old short-statured girl (figure 1) presented thyroidism prompted investigations for a mitochon- with seizures, progressive cataracts, and tetany. Fur- drial disorder,2 but a muscle biopsy was normal. ther evaluation revealed positive Chvostek sign (video), cerebrostriothalamic calcifications or Fahr syndrome, persistent hypocalcemia (total serum cal- REFERENCES cium 6.5 [normal 9–10.5] mg/100 mL), and pri- 1. Ahmed MA, Martinez A, Mariam S, Whitehouse W. mary hypoparathyroidism (serum parathyroid Chvostek’s sign and hypocalcaemia in children with sei- hormone Ͻ3.0 [normal 12–72] pg/mL). zures. Seizure 2004;13:217–222. 2. Tengan CH, Kiyomoto BH, Rocha MS, Tavares VL, Gabbai Chvostek sign, seen in hypocalcemia, hypomag- AA, Moraes CT. -

Qtc Prolongation Is Associated with Hypokalemia and Hypocalcemia in Emergency Department Patients Lucy Franjic Washington University School of Medicine in St
Washington University School of Medicine Digital Commons@Becker Division of Emergency Medicine/Emergency Care Conference Abstracts and Posters Research Section 2012 QTc prolongation is associated with hypokalemia and hypocalcemia in emergency department patients Lucy Franjic Washington University School of Medicine in St. Louis Stacey House Washington University School of Medicine in St. Louis Irena Vitkovitsky Washington University School of Medicine in St. Louis S. Eliza Halcomb Washington University School of Medicine in St. Louis Follow this and additional works at: http://digitalcommons.wustl.edu/em_conf Recommended Citation Franjic, Lucy; House, Stacey L.; Vitkovitsky, Irena; Halcomb, S. Eliza, "QTc prolongation is associated with hypokalemia and hypocalcemia in emergency department patients" (2012). Conference Abstracts and Posters. Paper 12. http://digitalcommons.wustl.edu/em_conf/12 This Presentation Paper is brought to you for free and open access by the Division of Emergency Medicine/Emergency Care Research Section at Digital Commons@Becker. It has been accepted for inclusion in Conference Abstracts and Posters by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. QTc Prolongation is Associated with Hypokalemia and Hypocalcemia in Emergency Department Patients Lucy Franjic, MD Stacey L. House MD PhD, Lucy Franjic MD, Irena Vitkovitsky MD, S. Eliza Halcomb MD Washington University in St. Louis Division of Emergency Medicine Society for Academic Emergency Medicine Great Plains Regional Research Forum St. Louis, MO. September 2012 © Stacey House, 2012 QTc Prolongation Congenital Six types (LQT1-LQT6) Mutations in genes encoding potassium and sodium transmembrane channel proteins Acquired Hypokalemia, hypocalcemia, hypomagnesemia, HIV, myocardial ischemia, numerous medications and drugs (i.e. -

Hypocalcemia: What a Surgeon Should Know 1Anish Kolly, 2Vijaya Sarathi, 3Sapana Bothra, 4Aromal Chekavar, 5Mayilvaganan Sabaretnam, 6Amit Agarwal
WJOES Anish Kolly et al 10.5005/jp-journals-10002-1215 HOW WE DO IT Hypocalcemia: What a Surgeon should know 1Anish Kolly, 2Vijaya Sarathi, 3Sapana Bothra, 4Aromal Chekavar, 5Mayilvaganan Sabaretnam, 6Amit Agarwal ABSTRACT of surgery, and differences in indication for surgery 5 Hypocalcemia is one of the sequelae following thyroidectomy across studies contribute to this wide variability. On the (TT) and becomes a complication when it becomes permanent. contrary, the risk of permanent postoperative hypocalce- Parathyroid preservation is a crucial step in the skillful opera- mia, which is defined as persistence of hypocalcemia even tive procedure of TT. When due care is not taken, the surgeon after 12 months of cervical surgery, has been reported to and the treating physician are faced with the issue of treating 6-9 the dreaded complication of permanent hypocalcemia. In this range from 0.6 to 12.1%. article, we address the issue of hypocalcemia following thyroid surgery and its management. ETIOPATHOGENESIS Keywords: Hypocalcemia, Parathyroid, Thyroidectomy. The cause of hypocalcemia after thyroid surgery is mul- How to cite this article: Kolly A, Sarathi V, Bothra S, tifactorial.10 Hypoparathyroidism seen after surgery can Chekavar A, Sabaretnam M, Agarwal A. Hypocalcemia: What be due to iatrogenic surgical trauma to the parathyroid a Surgeon should know. World J Endoc Surg 2017;9(2):72-77. glands or its blood supply or inadvertent removal of para- Source of support: Nil thyroid glands. Depending on the extent of parathyroid Conflict of interest: None gland damage, hypocalcemia may be transient, resolving within a few months, or permanent, in which the patient would require lifelong calcium and vitamin D supple- INTRODUCTION mentation. -

Electrolytes: Enteral and Intravenous – Adult – Inpatient Clinical Practice Guideline
Electrolytes: Enteral and Intravenous – Adult – Inpatient Clinical Practice Guideline Note: Active Table of Contents – Click each header below to jump to the section of interest Table of Contents INTRODUCTION .................................................................................................................................. 6 DEFINITIONS ....................................................................................................................................... 6 RECOMMENDATIONS ........................................................................................................................ 7 1. POTASSIUM (K+) ............................................................................................................................. 7 3- 2. PHOSPHATE (PO4 ) ........................................................................................................................ 9 3. MAGNESIUM (MG2+) ...................................................................................................................... 10 4. CALCIUM (CA2+) ............................................................................................................................ 12 5. SLIDING SCALE ELECTROLYTES ............................................................................................... 14 METHODOLOGY ............................................................................................................................... 15 COLLATERAL TOOLS & RESOURCES .......................................................................................... -

Hyperkalemia and Hypocalcemia - Literature Review Modalidades De Parada Cardíaca Induzida: Hipercalemia E Hipocalcemia - Revisão De Literatura
Oliveira MAB,SPECIAL et al. - Modes ARTICLE of induced cardiac arrest: hyperkalemia and Rev Bras Cir Cardiovasc 2014;29(3):432-6 hypocalcemia - Literature review Modes of induced cardiac arrest: hyperkalemia and hypocalcemia - Literature review Modalidades de parada cardíaca induzida: hipercalemia e hipocalcemia - revisão de literatura Marcos Aurélio Barboza de Oliveira1, MD, MsC; Antônio Carlos Brandi2, MD; Carlos Alberto dos Santos2, MD; Paulo Henrique Husseini Botelho2, MD; José Luis Lasso Cortez3, MD; Domingo Marcolino Braile4, MsC, PhD DOI: 10.5935/1678-9741.20140074 RBCCV 44205-1572 Abstract Resumo The entry of sodium and calcium play a key effect on myocyte A entrada de sódio e cálcio desempenham efeito chave no miócito subjected to cardiac arrest by hyperkalemia. They cause cell submetido à parada cardíaca por hiperpotassemia. Eles provocam swelling, acidosis, consumption of adenosine triphosphate and edema celular, acidose, consumo de trifosfato de adenosina e desen- trigger programmed cell death. Cardiac arrest caused by hypo- cadeiam processo de morte celular programada. A parada cardíaca calcemia maintains intracellular adenosine triphosphate levels, provocada por hipocalcemia mantém os níveis intracelulares de improves diastolic performance and reduces oxygen consumption, trifosfato de adenosina, melhora o rendimento diastólico e reduz o which can be translated into better protection to myocyte injury consumo de oxigênio, o que pode ser traduzido em melhor proteção induced by cardiac arrest. do miócito às lesões provocadas pela parada cardíaca induzida. Descriptors: Heart Arrest, Induced. Myocardial Ischemia. Descritores: Parada Cardíaca Induzida. Isquemia Miocárdi- Hyperkalemia. Hypocalcemia. ca. Hiperpotassemia. Hipocalcemia. INTRODUCTION to approach intracardiac diseases[3] by using substances that trigger controlled cardiac arrest, commonly known as cardio- The first cardiac surgeries were performed on a beating plegic agents [4]. -

Chapter 73 1009
C h a p t e r 73 Fluid and Electrolyte Issues in Pediatric Critical Illness Robert Lynch, Ellen Glenn Wood, and Tara M. Neumayr intraoperative fluids influence acid-base and electrolyte status, PEARLS particularly of vulnerable patients.4 The choice of Na+ content • Hypotonic maintenance IV fluids are associated with mild to and balancing ions of IVF for postoperative or critical care moderate hyponatremia in postoperative patients. maintenance may be important for some patients thus justify- Anesthesia, stress, and inflammatory mediators probably ing additional expense. Clearly 0.18 and 0.225 mM saline is 5,6 contribute. Electrolyte monitoring in patients at risk is associated with a higher incidence of mild hyponatremia, essential for detection and management of the occasional although controlled trials do not show this effect for 0.46 mM 7,8 patient who develops severe hyponatremia. This critical saline. Severe hyponatremia has been associated with pul- effect of the syndrome of inappropriate antidiuretic hormone monary or CNS illness in pediatrics and is infrequent even may occur even in patients on isotonic IV fluids. within those categories, with the exception of children with • Medical patients with high levels of inflammatory mediators traumatic brain injury (TBI). Among patients with those and appear to be at increased risk of significant hyponatremia. other illnesses, the evolving study of the influence of inflam- Interleukin effects on antidiuretic hormone release may matory mediators directly on the hypothalamus and indirectly contribute. on vasopressin secretion may further clarify which patients are 9,10 • Albumin infusions have been generally safe but may at most risk of clinically significant hyponatremia. -

Hypomagnesemia with Hypocalcemia and Vitamin D Deficiency with Secondary Hypoparathyroidism
Case History Clinical Case Reports International Published: 22 Sep, 2020 Hypomagnesemia with Hypocalcemia and Vitamin D Deficiency with Secondary Hypoparathyroidism Bharat Parmar1* and Jwal Bharat2 1Department of Pediatrics, Zydus Medical College and Hospital, India 2Department of Pediatrics, Long Island University, USA Abstract The divalent cations, calcium and magnesium play vital role in neuromuscular function and cellular metabolism. Hypocalcemia related to parathyroid disorder, renal failure and bone disorders. The recent availability of ionized calcium measurement is helping to increase the accuracy of detection of this state. Evidence suggests that hypomagnesemia is a significant presence of refractory hypocalcemia, particularly in critical ill patient, with clinical significance relating mainly to concomitant electrolyte deficiency such hypokalemia. Case History A 10 year old child has came with generalized tonic-clonic convulsion first episode no history aura, loss of consciousness, bowel bladder incontinence was associated with up-rolling of eye balls, no froth from mouth no post seizure weakness or confusion, no history of precipitating factors dehydration, fever. Convulsion lasted for 4 min, normal birth history, and no similar episode in past, no history of convulsion in family, immunized up to age and normal development history. Past history of frequent spasms of hand peri-oral tingling, numbness. On clinical examination Chvostek’s sign and Trousseaus sign positive. Examination of central nervous system and endocrine was unremarkable, other system no abnormality, no abnormality in echocardiogram. Investigations Serum calcium 6.35 mg/dl, (8.4 mg/dl to 10.4 mg/dl), Ionized calcium 3.7 meq/l (4.3 meq/l to 5.3 meq/l), magnesium 1.2 mg/dl (1.7 mg/dl to 2.4 mg/dl), Serum alkaline phosphatase ALP 340 µ/L, Inorganic phosphorus 4.2 mg/dl (2.4 mg/dl to 4.5 mg/dl) serum albumin 3.75 gm/dl (3.2 gm/ OPEN ACCESS dl to 5 gm/dl). -

Fluid & Electrolyte Disorders
FLUID & ELECTROLYTE DISORDERS 11/2/18 Don Beckstead M.D. 63WYCH Disclosures • I have nothing to disclose • This talk is intended to cover adult electrolyte issues 2 63WYCH GOALS AND OBJECTIVES • Identify causes of common electrolyte abnormalities found in primary care office patients. • Discuss signs and symptoms found in patients who have common electrolyte abnormalities. • Become comfortable with treatment modalities that can be used to correct common electrolyte abnormalities. 3 63WYCH 1 ELECTROLYTES • We will cover: –High and low sodium –High and low potassium –High and low calcium –High and low magnesium 4 63WYCH SOME BASIC PRINCIPLES • Kidneys prioritize fluid and electrolyte balance at the possible expense of acid‐base balance. • Normally functioning kidneys have a great capacity to handle increased or decreased intake of most electrolytes. • Most electrolyte abnormalities found are in asymptomatic patients. • Sodium abnormalities are usually actually water abnormalities 5 Text 63WYCH to 828-216-8114 BASIC METABOLIC PROFILE . Sodium (Na+ ) 136‐150 . Chloride (Cl‐) 100‐110 . Bicarbonate (CO2) 22‐28 . Potassium (K+) 3.6‐5.0 . BUN (blood urea nitrogen) 5‐18 . Creatinine 0.6 ‐ 1.3 . Anion Gap (Na+‐ {Cl‐ + CO2}) = ~ 12 . Calcium (Ca++) 8.5 – 10.3 . Magnesium (Mg++) 1.5 ‐ 2.3 6 2 SODIUM 7 63WYCH HYPONATREMIA CAUSES • Suppressed ADH – CKD – Polydipsia • Increased ADH – CHF – Cirrhosis – Thiazide diuretics – SIADH – Pregnancy/hypothyroidism/adrenal insufficiency 8 63WYCH HYPONATREMIA w/ HIGH/NL OSMO • Hyperlipidemia • Hyperproteinemia • Mannitol administration • Hyperglycemia • CRF (BUN ineffective osmol) 9 63WYCH 3 HYPONATREMIA SYMPTOMS • Usually none • If not pseudohyponatremia (+ low osmolality), then symptoms are usually related to development of cerebral edema – Nausea/vomiting – Malaise/lethargy – Headache – Seizures/coma/respiratory arrest 10 63WYCH HYPONATREMIA WORK‐UP • Urine osmol. -
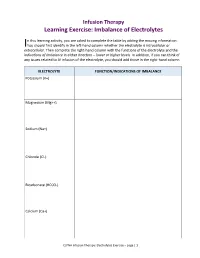
Learning Exercise: Imbalance of Electrolytes
Infusion Therapy Learning Exercise: Imbalance of Electrolytes n this learning activity, you are asked to complete the table by adding the missing information. I You should first identify in the left-hand column whether the electrolyte is intracellular or extracellular. Then complete the right-hand column with the functions of the electrolyte and the indications of imbalance in either direction – lower or higher levels. In addition, if you can think of any issues related to IV infusion of the electrolyte, you should add those in the right-hand column. ELECTROLYTE FUNCTION/INDICATIONS OF IMBALANCE Potassium (K+) Magnesium (Mg++) Sodium (Na+) Chloride (Cl-) Bicarbonate (HCO3-) Calcium (Ca+) CLPNA Infusion Therapy: Electrolytes Exercise – page | 1 Answers for Electrolyte Table Potassium (K+) (Intracellular) The distribution of potassium between the intracellular and extracellular compartments regulates electrical membrane potentials controlling the excitability of nerve and muscle cells as well as the contractility of skeletal, cardiac, and smooth muscle tissue. When levels of potassium are low (hypokalemia), signs and symptoms include dizziness, muscle weakness, leg cramps, cardiac arrhythmia, hypotension, thirst, nausea, anorexia, poorly concentrated urine, [and] polyuria. When levels of potassium are high (hyperkalemia), signs and symptoms include nausea and vomiting, intestinal cramps, diarrhea, paresthesias, weakness, dizziness, muscle cramps, changes in electrocardiogram, [and] risk of cardiac arrest with severe excess. Magnesium (Mg++) (Intracellular) Magnesium acts as a cofactor in many intracellular enzyme reactions…[and] is essential to all reactions that require ATP, for every step related to replication and transcription of DNA, and for the translation of messenger RNA…[and] is required for cellular energy metabolism. -

Hypercalcemia Colorado ACP Meeting February 2017 David Tanaka MD FACP Disclosures
Hypercalcemia Colorado ACP meeting February 2017 David Tanaka MD FACP Disclosures None Objectives • Be able to effectively and efficiently evaluate hypercalcemia • Be able to counsel patients on their diagnosis and prognosis • Be able to counsel patients on their options for treatment 60 yo woman – evaluation of fatigue • Anxiety, hypothyroidism, hypertension, obesity • Levothyroxine, venlafaxine, HCTZ, losartan • No recent illness • Some increased stress • Snores • Fatigue is worse for 1+ year but most noticeable for 1-2 months 60 yo woman – evaluation of fatigue • Weight is stable, exam is normal • Labs 9 months previous TSH 4.16, prior 2.97 • Glucose 84, creatinine 0.66, CBC and LFT’s normal • Calcium 10.6 What do you order to evaluate her hypercalcemia • Calcium, ionized calcium, PTH, PTHrp, Phosphate, creatinine, vitamin D(25-OH and 1,25 OH), 24 hr urine calcium and creatinine • Calcium, phosphate, creatinine, PTH • Calcium, creatinine, PTH, vitamin D (25OH) • All of the above + sestamibi scan of neck Etiology and Differentiation of Hypercalcemia Calcium PTH Vitamin D Primary Hyperparathyroidism Elevated High normal or elevated Normal or low Malignancy Elevated Low (<20 ng/dl) Depends on malignancy 60 yo woman – lab evaluation • Calcium 11.1, Creatinine 0.65, PTH 73 (12-88) • 24 hour urine Calcium 3.0 mg/dl X 1266 cc = 38 mg / d • 24 hour urine Creatinine 118 mg / dl X 1266 = 1481 mg / d 60 yo woman - differential diagnosis • Primary hyperparathyroidism • Hydrochlorothiazide causing hypercalcemia • Familial hypocalciuric hypercalcemia • All of the above Familial hypocalciuric hypercalcemia • Autosomal dominant – caused by inactivating mutations in the gene for calcium-sensing receptor (CaSR or CaR) • Parathyroid gland is less sensitive to calcium, higher calcium concentration is required to reduce PTH.