051800 Hypernatremia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyponatremia and Hypernatremia MICHAEL M
This is a corrected version of the article that appeared in print. Diagnosis and Management of Sodium Disorders: Hyponatremia and Hypernatremia MICHAEL M. BRAUN, DO, Madigan Army Medical Center, Tacoma, Washington CRAIG H. BARSTOW, MD, Womack Army Medical Center, Fort Bragg, North Carolina NATASHA J. PYZOCHA, DO, Madigan Army Medical Center, Tacoma, Washington Hyponatremia and hypernatremia are common findings in the inpatient and outpatient settings. Sodium disorders are associated with an increased risk of morbidity and mortality. Plasma osmolality plays a critical role in the patho- physiology and treatment of sodium disorders. Hyponatremia and hypernatremia are classified based on volume status (hypovolemia, euvolemia, and hypervolemia). Sodium disorders are diagnosed by findings from the history, physical examination, laboratory studies, and evaluation of volume status. Treatment is based on symptoms and underlying causes. In general, hyponatremia is treated with fluid restriction (in the setting of euvolemia), isotonic saline (in hypovolemia), and diuresis (in hypervolemia). A combination of these therapies may be needed based on the presentation. Hypertonic saline is used to treat severe symptomatic hyponatremia. Medications such as vaptans may have a role in the treatment of euvolemic and hypervolemic hyponatremia. The treatment of hypernatremia involves correcting the underlying cause and correcting the free water deficit. Am( Fam Physician. 2015;91(5):299-307. Copy- right © 2015 American Academy of Family Physicians.) More online yponatremia is a common elec- a worse prognosis in patients with liver cir- at http://www. trolyte disorder defined as a rhosis, pulmonary hypertension, myocardial aafp.org/afp. serum sodium level of less than infarction, chronic kidney disease, hip frac- CME This clinical content 135 mEq per L.1-3 A Dutch sys- tures, and pulmonary embolism.1,8-10 conforms to AAFP criteria Htematic review of 53 studies showed that the for continuing medical Etiology and Pathophysiology education (CME). -

Hyperchloremia – Why and How
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):347–353 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Brief review Hyperchloremia – Why and how Glenn T. Nagami Nephrology Section, Department of Medicine, VA Greater Los Angeles Healthcare System and David Geffen School of Medicine at UCLA, United States a r t i c l e i n f o a b s t r a c t Article history: Hyperchloremia is a common electrolyte disorder that is associated with a diverse group of Received 5 April 2016 clinical conditions. The kidney plays an important role in the regulation of chloride concen- Accepted 11 April 2016 tration through a variety of transporters that are present along the nephron. Nevertheless, Available online 3 June 2016 hyperchloremia can occur when water losses exceed sodium and chloride losses, when the capacity to handle excessive chloride is overwhelmed, or when the serum bicarbonate is low Keywords: with a concomitant rise in chloride as occurs with a normal anion gap metabolic acidosis Hyperchloremia or respiratory alkalosis. The varied nature of the underlying causes of the hyperchloremia Electrolyte disorder will, to a large extent, determine how to treat this electrolyte disturbance. Serum bicarbonate Published by Elsevier Espana,˜ S.L.U. on behalf of Sociedad Espanola˜ de Nefrologıa.´ This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/). -

An Infant with Chronic Hypernatremia
European Journal of Endocrinology (2006) 155 S141–S144 ISSN 0804-4643 An infant with chronic hypernatremia M L Marcovecchio Department of Paediatrics, University of Chieti, Via dei Vestini 5, 66100 Chieti, Italy (Correspondence should be addressed to M L Marcovecchio; Email: [email protected]) Abstract A 4-month-old boy was presented with failure to thrive, refusal to feed, delayed motor development, truncal hypotonia, and head lag. His plasma osmolality and sodium were significantly high, while his urine osmolality was inappropriately low and did not increase after desmopressin administration. Despite his hyperosmolality, he presented with a lack of thirst and became clearly polyuric and polydipsic only at the age of 2 years. Initial treatment with indomethacin was ineffective, while the combination of hydrochlorothiazide and amiloride was effective and well tolerated. European Journal of Endocrinology 155 S141–S144 Introduction (61 ml/kg) respectively. Other investigations including thyroid and adrenal function were normal. He was Chronic hypernatremia in young patients is generally refusing oral fluids despite being hypernatremic. the result of alterations in the mechanisms controlling A cranial magnetic resonance imaging scan excluded fluid balance (1). Excessive water loss, as in diabetes structural abnormalities in the hypophysis, hypo- insipidus, or an inadequate fluid intake, as in adipsic thalamus and surrounding area. There was no response hypernatremia, may be the underlying cause. The to 0.3 mg 1-desamino-8-D-arginine-vasopressin (DDAVP) differential diagnosis between these conditions is given intravenously (osmolality pre- 374 mosmol/kg; important in order to choose the appropriate treatment. post- 371 mosmol/kg). This suggested a tubular defect However, pitfalls in the diagnosis are often related to an causing nephrogenic diabetes insipidus (NDI). -

Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside Or Colistin Therapy: a Systematic Review
antibiotics Review Electrolyte and Acid-Base Disorders Triggered by Aminoglycoside or Colistin Therapy: A Systematic Review Martin Scoglio 1,* , Gabriel Bronz 1, Pietro O. Rinoldi 1,2, Pietro B. Faré 3,Céline Betti 1,2, Mario G. Bianchetti 1, Giacomo D. Simonetti 1,2, Viola Gennaro 1, Samuele Renzi 4, Sebastiano A. G. Lava 5 and Gregorio P. Milani 2,6,7 1 Faculty of Biomedicine, Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] (G.B.); [email protected] (P.O.R.); [email protected] (C.B.); [email protected] (M.G.B.); [email protected] (G.D.S.); [email protected] (V.G.) 2 Department of Pediatrics, Pediatric Institute of Southern Switzerland, Ospedale San Giovanni, Ente Ospedaliero Cantonale, 6500 Bellinzona, Switzerland; [email protected] 3 Department of Internal Medicine, Ospedale La Carità, Ente Ospedaliero Cantonale, 6600 Locarno, Switzerland; [email protected] 4 Division of Hematology and Oncology, The Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected] 5 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1011 Lausanne, Switzerland; [email protected] 6 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy 7 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected] Citation: Scoglio, M.; Bronz, G.; Abstract: Aminoglycoside or colistin therapy may alter the renal tubular function without decreasing Rinoldi, P.O.; Faré, P.B.; Betti, C.; the glomerular filtration rate. This association has never been extensively investigated. -

A Case of Hypocalciuric Hypercalcemia Accompanying Cystic Fibrosis
J Clin Res Pediatr Endocrinol 2015;7(Suppl 2):77-92 A Case of Hypocalciuric Hypercalcemia Accompanying Cystic Fibrosis Yaşar Şen, Sevil Arı Yuca, Fuat Buğrul Blood and urine tests were done in order to find the etiology of hypercalcemia (PTH: 65.5 pg/mL, 25-hydroxy vitamin Selçuk University Faculty of Medicine, Department of Pediatric D: 43.5 ng/mL, urine Ca/Cr ratio 0.19, urine Ca clearance Endorcinology, Konya, Turkey 0.004). In the CaSR gene mutation study, A986S and R990G polymorphisms were heterozygous. 1 month after hydration Introduction: Familial hypocalciuric hypercalcemia (FHH) and clinical state being back to normal, Ca levels were is an autosomal dominant disorder which occurs by an in normal range. In stressful situations, he experienced inactivating gene mutation in the calcium (Ca)-sensing hypernatremia and hypercalcemia together. receptor gene (CaSR). Prevalence is estimated at 1: 78000. Discussion: Polymorphisms may change protein function Ca regulates parathormone (PTH) secretion via CaSR on and capacity of one to repair its damaged DNA. Genetic parathyroid cells. Low levels of Ca increases PTH secretion. polymorphisms help us to define personal sensitivities to CaSR mutation that causes loss of function, leads to total some diseases. The most common CaSR polymorphisms or partial insensitivity of parathyroid cells to Ca’s inhibitory are A986S, R990G, Q1011E and A826T. Our patient effect. For this reason, in order to suppress Ca’s PTH had A986S and R990G mutations. Heterozygous CaSR secretory effect, the setpoint is raised. A higher blood Ca gene mutations generally cause mild disorders in clinic. level is needed to suppress the PTH secretion. -

Non-Accidental Salt Poisoning
448 ArchivesofDiseasein Childhood 1993; 68: 448-452 Non-accidental salt poisoning Roy Meadow Arch Dis Child: first published as 10.1136/adc.68.4.448 on 1 April 1993. Downloaded from Abstract Table I Presentation of12 childrenfrom 10families The clinical features of 12 children who Sex (M/F) 6/6 incurred non-accidental salt poisoning are Age (months) of first confirmed hypernatraemia reported. The children usually presented to 11 children I 5-9 (median 2 - 5) 1 child 41 hospital in the first six months of life with Duration (months) of recurrent unexplained hypernatraemia and associated hypernatraemia 1-45 (median 3) illness. Most ofthe children suffered repetitive poisoning before detection. The perpetrator was believed to be the mother for 10 children, the mother confessed to the poisoning and the father for one, and either parent for one. explained how she had done it.) Four children had serum sodium concentra- tions above 200 mmol/l. Seven children had incurred other fabricated illness, drug inges- PRESENTATION tion, physical abuse, or failure to thrive/ The main features are outlined in table 1. neglect. Two children died; the other 10 Usually the child was presented to hospital remained healthy in alternative care. Features within the first three months of life because of are described that should lead to earlier repetitive illness. Vomiting was the predominant detection of salt poisoning; the importance of feature, often associated with diarrhoea and checking urine sodium excretion, whenever failure to thrive. At times there was drowsiness hypernatraemia occurs, is stressed. which, on occasion, could amount to coma. Four (Arch Dis Child 1993; 68: 448-452) children had markedly abnormal neurological signs including rigidity, hyper-reflexia, and seizures at the time of hypernatraemia. -

Neurologic Complications of Electrolyte Disturbances and Acid–Base Balance
Handbook of Clinical Neurology, Vol. 119 (3rd series) Neurologic Aspects of Systemic Disease Part I Jose Biller and Jose M. Ferro, Editors © 2014 Elsevier B.V. All rights reserved Chapter 23 Neurologic complications of electrolyte disturbances and acid–base balance ALBERTO J. ESPAY* James J. and Joan A. Gardner Center for Parkinson’s Disease and Movement Disorders, Department of Neurology, UC Neuroscience Institute, University of Cincinnati, Cincinnati, OH, USA INTRODUCTION hyperglycemia or mannitol intake, when plasma osmolal- ity is high (hypertonic) due to the presence of either of The complex interplay between respiratory and renal these osmotically active substances (Weisberg, 1989; function is at the center of the electrolytic and acid-based Lippi and Aloe, 2010). True or hypotonic hyponatremia environment in which the central and peripheral nervous is always due to a relative excess of water compared to systems function. Neurological manifestations are sodium, and can occur in the setting of hypovolemia, accompaniments of all electrolytic and acid–base distur- euvolemia, and hypervolemia (Table 23.2), invariably bances once certain thresholds are reached (Riggs, reflecting an abnormal relationship between water and 2002). This chapter reviews the major changes resulting sodium, whereby the former is retained at a rate faster alterations in the plasma concentration of sodium, from than the latter (Milionis et al., 2002). Homeostatic mech- potassium, calcium, magnesium, and phosphorus as well anisms protecting against changes in volume and sodium as from acidemia and alkalemia (Table 23.1). concentration include sympathetic activity, the renin– angiotensin–aldosterone system, which cause resorption HYPONATREMIA of sodium by the kidneys, and the hypothalamic arginine vasopressin, also known as antidiuretic hormone (ADH), History and terminology which prompts resorption of water (Eiskjaer et al., 1991). -

Disorders of Sodium and Water Balance
Disorders of Sodium and Water Balance Theresa R. Harring, MD*, Nathan S. Deal, MD, Dick C. Kuo, MD* KEYWORDS Dysnatremia Water balance Hyponatremia Hypernatremia Fluids for resuscitation KEY POINTS Correct hypovolemia before correcting sodium imbalance by giving patients boluses of isotonic intravenous fluids; reassess serum sodium after volume status normalized. Serum and urine electrolytes and osmolalities in patients with dysnatremias in conjunction with clinical volume assessment are especially helpful to guide management. If an unstable patient is hyponatremic, give 2 mL/kg of 3% normal saline (NS) up to 100 mL over 10 minutes; this may be repeated once if the patient continues to be unstable. If unstable hypernatremic patient, give NS with goal to decrease serum sodium by 8 to 15 mEq/L over 8 hours. Correct stable dysnatremias no faster than 8 mEq/L to 12 mEq/L over the first 24 hours. INTRODUCTION Irregularities of sodium and water balance most often occur simultaneously and are some of the most common electrolyte abnormalities encountered by emergency med- icine physicians. Approximately 10% of all patients admitted from the emergency department suffer from hyponatremia and 2% suffer from hypernatremia.1 Because of the close nature of sodium and water balance, and the relatively rigid limits placed on the central nervous system by the skull, it is not surprising that most symptoms related to disorders of sodium and water imbalance are neurologic and can, therefore, be devastating. Several important concepts are crucial to the understanding of these disorders, the least of which include body fluid compartments, regulation of osmo- lality, and the need for rapid identification and appropriate management. -

Evaluating and Managing Electrolyte Disbalances in the Outpatient Setting Disclosure
EVALUATING AND MANAGING ELECTROLYTE DISBALANCES IN THE OUTPATIENT SETTING DISCLOSURE There are no conflicts of interest Homeostasis • Potassium • Water Evaluation and Management of Electrolyte TO BE DISCUSSED Disbalances • Hyperkalemia • Hypokalemia • Hypernatremia • Hyponatremia Summary DISORDERS OF POTASSIUM BALANCE: HYPERKALEMIA AND HYPOKALEMIA POTASSIUM HOMEOSTASIS • Aldosterone • High Na+ delivery to distal tubule Increase (diuretics) Renal K+ • High urine flow (osmotic diuresis) Excretion • High serum K+ level • Delivery bicarbonate to distal tubule • Absence, or very low aldosterone • Low Na+ delivery to the distal Decrease tubule Renal K+ • Low urine flow Excretion • Low serum K+ level • Kidney Injury MORTALITY IN DYSKALEMIA Collins et al Am J Nephrol 2017;46:213- 221 HYPERKALEMIA MY PATIENT HAS HYPERKALEMIA, WHAT SHOULD I DO? H&P and Check other medication causes •Changes? Treat review •Send home •Symptoms? Treat •Metabolic acidosis •Obstruction •Hyperosmolarity •K+ increasing meds •CKD Pseudohyperkale EKG and BMP mia CAUSES OF HYPERKALEMIA Increased potassium release from Reduced urinary potassium excretion cells • Pseudohyperkalemia • Acute and chronic kidney disease • Fist clenching • Reduced aldosterone secretion or • Tourniquet use response to aldosterone • Metabolic acidosis • Reduced distal sodium and water • Insulin deficiency, hyperglycemia, delivery and hyperosmolality • Drugs • Increased tissue catabolism • Drugs • Hyperkalemic Periodic Palasysis Biff Palmer A Physiologic-Based Approach to the Evaluation of a Patient -

Electrolyte Imbalance
Electrolyte Imbalance For Providers Myth: Volume depletion and electrolyte imbalance are interchangeable terms. Fact: While we often use these terms interchangeably, they are actually different conditions and require different treatment. Volume depletion is the loss of salt and water. Residents with volume depletion may show changes to the BUN and creatinine or present with new orthostatic hypotension. Treatment of volume depletion requires replacement of both sodium and water, usually with normal saline. Electrolyte imbalance is loss of water only. In true dehydration, the sodium is always elevated. Treatment of residents with electrolyte imbalance requires replacement of water deficit with oral water (mild electrolyte imbalance) or with intravenous fluids. Giving a patient with true electrolyte imbalance (hypernatremia) normal saline is likely to worsen their condition. Myth: Electrolyte imbalance can be diagnosed by physical exam. Fact: While there are some signs on physical exam that may lead you to think about electrolyte imbalance such as dry tongue or dry axilla, these are nonspecific. The diagnosis of electrolyte imbalance is a laboratory diagnosis. You need to get at least a BMP. Dehydration is diagnosed by hypernatremia (deficit of free water). Volume depletion is diagnosed by changes to the BUN and creatinine. Myth: If a patient has a G-tube, then I don’t need to place an IV to hydrate them. Fact: For residents with mild to moderate electrolyte imbalance, you may be able to hydrate them through their g-tube. However, for severe electrolyte imbalance or volume depletion, you likely still need to place an IV as the ability of the gut to absorb fluids will limit your ability to replenish fluids quickly enough for these residents. -

Fluid & Electrolyte Disorders
FLUID & ELECTROLYTE DISORDERS 11/2/18 Don Beckstead M.D. 63WYCH Disclosures • I have nothing to disclose • This talk is intended to cover adult electrolyte issues 2 63WYCH GOALS AND OBJECTIVES • Identify causes of common electrolyte abnormalities found in primary care office patients. • Discuss signs and symptoms found in patients who have common electrolyte abnormalities. • Become comfortable with treatment modalities that can be used to correct common electrolyte abnormalities. 3 63WYCH 1 ELECTROLYTES • We will cover: –High and low sodium –High and low potassium –High and low calcium –High and low magnesium 4 63WYCH SOME BASIC PRINCIPLES • Kidneys prioritize fluid and electrolyte balance at the possible expense of acid‐base balance. • Normally functioning kidneys have a great capacity to handle increased or decreased intake of most electrolytes. • Most electrolyte abnormalities found are in asymptomatic patients. • Sodium abnormalities are usually actually water abnormalities 5 Text 63WYCH to 828-216-8114 BASIC METABOLIC PROFILE . Sodium (Na+ ) 136‐150 . Chloride (Cl‐) 100‐110 . Bicarbonate (CO2) 22‐28 . Potassium (K+) 3.6‐5.0 . BUN (blood urea nitrogen) 5‐18 . Creatinine 0.6 ‐ 1.3 . Anion Gap (Na+‐ {Cl‐ + CO2}) = ~ 12 . Calcium (Ca++) 8.5 – 10.3 . Magnesium (Mg++) 1.5 ‐ 2.3 6 2 SODIUM 7 63WYCH HYPONATREMIA CAUSES • Suppressed ADH – CKD – Polydipsia • Increased ADH – CHF – Cirrhosis – Thiazide diuretics – SIADH – Pregnancy/hypothyroidism/adrenal insufficiency 8 63WYCH HYPONATREMIA w/ HIGH/NL OSMO • Hyperlipidemia • Hyperproteinemia • Mannitol administration • Hyperglycemia • CRF (BUN ineffective osmol) 9 63WYCH 3 HYPONATREMIA SYMPTOMS • Usually none • If not pseudohyponatremia (+ low osmolality), then symptoms are usually related to development of cerebral edema – Nausea/vomiting – Malaise/lethargy – Headache – Seizures/coma/respiratory arrest 10 63WYCH HYPONATREMIA WORK‐UP • Urine osmol. -
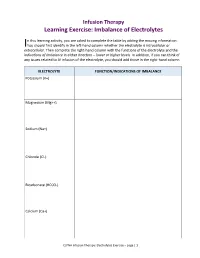
Learning Exercise: Imbalance of Electrolytes
Infusion Therapy Learning Exercise: Imbalance of Electrolytes n this learning activity, you are asked to complete the table by adding the missing information. I You should first identify in the left-hand column whether the electrolyte is intracellular or extracellular. Then complete the right-hand column with the functions of the electrolyte and the indications of imbalance in either direction – lower or higher levels. In addition, if you can think of any issues related to IV infusion of the electrolyte, you should add those in the right-hand column. ELECTROLYTE FUNCTION/INDICATIONS OF IMBALANCE Potassium (K+) Magnesium (Mg++) Sodium (Na+) Chloride (Cl-) Bicarbonate (HCO3-) Calcium (Ca+) CLPNA Infusion Therapy: Electrolytes Exercise – page | 1 Answers for Electrolyte Table Potassium (K+) (Intracellular) The distribution of potassium between the intracellular and extracellular compartments regulates electrical membrane potentials controlling the excitability of nerve and muscle cells as well as the contractility of skeletal, cardiac, and smooth muscle tissue. When levels of potassium are low (hypokalemia), signs and symptoms include dizziness, muscle weakness, leg cramps, cardiac arrhythmia, hypotension, thirst, nausea, anorexia, poorly concentrated urine, [and] polyuria. When levels of potassium are high (hyperkalemia), signs and symptoms include nausea and vomiting, intestinal cramps, diarrhea, paresthesias, weakness, dizziness, muscle cramps, changes in electrocardiogram, [and] risk of cardiac arrest with severe excess. Magnesium (Mg++) (Intracellular) Magnesium acts as a cofactor in many intracellular enzyme reactions…[and] is essential to all reactions that require ATP, for every step related to replication and transcription of DNA, and for the translation of messenger RNA…[and] is required for cellular energy metabolism.