Animal-Environment Interface at the University of Veterinary Medicine Hannover
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Siedenburg, Flecken, 032515407034
Gebäude und Wohnungen sowie Wohnverhältnisse der Haushalte Gemeinde Siedenburg, Flecken am 9. Mai 2011 Ergebnisse des Zensus 2011 Zensus 9. Mai 2011 Siedenburg, Flecken (Landkreis Diepholz) Regionalschlüssel: 032515407034 Seite 2 von 32 Zensus 9. Mai 2011 Siedenburg, Flecken (Landkreis Diepholz) Regionalschlüssel: 032515407034 Inhaltsverzeichnis Einführung ................................................................................................................................................ 4 Rechtliche Grundlagen ............................................................................................................................. 4 Methode ................................................................................................................................................... 4 Systematik von Gebäuden und Wohnungen ............................................................................................. 5 Tabellen 1.1 Gebäude mit Wohnraum und Wohnungen in Gebäuden mit Wohnraum nach Baujahr, Gebäudetyp, Zahl der Wohnungen, Eigentumsform und Heizungsart .............. 6 1.2 Gebäude mit Wohnraum nach Baujahr und Gebäudeart, Gebäudetyp, Zahl der Wohnungen, Eigentumsform und Heizungsart ........................................................... 8 1.3.1 Gebäude mit Wohnraum nach regionaler Einheit und Baujahr, Gebäudeart, Gebäudetyp, Zahl der Wohnungen, Eigentumsform und Heizungsart ..................................... 10 1.3.2 Gebäude mit Wohnraum nach regionaler Einheit und Baujahr, Gebäudeart, Gebäudetyp, Zahl -

Titelseite Druckvorlage
Unser Dorf hat Zukunft Kreiswettbewerb 2014 Kreiswettbewerb 2014 Inhalt Seite Impressionen 2014 4 Vorwort des Landrats 6 Vorwort des Vorsitzenden 7 Teilnehmerdörfer und Platzierungen 8 Die Bewertungskommission 9 Die Bewertungskriterien 11 Das Bewertungsverfahren 19 Berichte der Kommissionsmitglieder Kreiswettbewerb Aschen 20 Heiligenloh 22 Sudwalde 25 Bahrenborstel 27 Ehrenburg 29 Ströhen 31 Freistatt 33 Gessel-Leerßen 35 Groß Lessen 38 Heiligenrode 40 Kirchdorf 42 Siedenburg 44 Varrel 46 Wachendorf 48 Kreiswettbewerb 2014 Inhalt (Fortsetzung) Seite Sonderwettbewerb für Bauernschaften und Weiler Staffhorst 50 Ridderade und Stophel 52 Wedehorn und Klövenhausen 54 Holzhausen 56 Albringhausen und Schorlingsborstel 58 Kuppendorf 60 Lindern 62 Nechtelsen 64 Scharringhausen 66 Schlahe und Bockhorn 68 Impressum: Landkreis Diepholz Fachdienst Kreisentwicklung Niedersachsenstr. 2 49356 Diepholz Tel.: 05441 / 976-1298 www.diepholz.de 3 Kreiswettbewerb 2014 Impressionen 2014 4 Kreiswettbewerb 2014 5 Kreiswettbewerb 2014 Vorwort des Landrates Die besten Erfolgsgeschichten wiederholen sich – wie der Wettwerb „Unser Dorf hat Zukunft“. Der Kreiswettbewerb wird im dreijährigen Turnus ausgetragen und fand in diesem Jahr zum 14. Mal in unserem Landkreis Diepholz statt. 24 Dörfer haben sich den Zukunftsthemen und den Herausforderungen des Wettbewerbs auf eindrucksvolle Weise gestellt. Der Weg, wie dieser Wettbewerb die Zukunftsthemen in die Dörfer transportiert, ist außergewöhnlich. In vielen Dörfern werden die Bewohnerinnen und Bewohner von jung bis alt mobilisiert. Männer, Frauen, Kinder und Jugendliche sitzen zusammen, machen Vorschläge, setzen Projekte um und schaffen so eine neue Form der Gemeinschaft. Keine Informationsveran- staltung, kein Rundschreiben, keine Broschüre zu Themen der ländlichen Zukunft, kann eine solche Wirkung entfalten. Deshalb kann ich im Namen des Landkreises nur jedem teilnehmenden Dorf gratulieren, das diese Chance ergriffen hat. -

The Upper Cretaceous Succession (Cenomanian- Santonian) of the Staffhorst Shaft, Lower Saxony, Northern Germany: Integrated Bios
Acta Geologica Polonica, Vol. 49 (1999), No.3, pp. 175-213 The Upper Cretaceous succession (Cenomanian Santonian) of the Staffhorst Shaft, Lower Saxony, northern Germany: integrated biostratigraphic, lithostratigraphic and downhole geophysical log data BIRGIT NIEBUHR 1, REINHARD BALDSCHUHN2, GUNDOLF ERNST3, IRENEUSZ WALASZCZYK4, WOLFGANG WEISS5 & CHRISTOPHER J. WOOD6 I Institut fiir Palaontologie, Bayerische lulills-Maximilians-Universitat, Pleicherwall I, D-97070 Wiirzburg, Germany. E-mail: [email protected] 2 Ernst-Pfliiger-Str. 7, D-30938 Burgdorf-Wettmar, Germany 3 Institutfiir Paliiontologie, Freie Universitat Berlin, Malteser Str. 74-100, D-12249 Berlin, Germany. E-mail: [email protected] 4 Institute of Geology, University of Warsaw, AI. Zwirki i Wigury 93, PL-02-089 Warszawa, Poland. E-mail: [email protected] 5 Bundesanstalt fur Geowissenschaften und Rohstoffe, Stilleweg 2, D-30655 Hannover, Germany. E-mail: [email protected] 6 Scops Geological Services Ltd., 20 Temple Road, Croydon, Surrey CRO I HT, United Kingdom. ABSTRACT: NIEBUHR, B., BALDSCHUHN, R., ERNST,G., WALASZCZYK, 1., WEISS, W. & WOOD, C.J. 1999. The Upper Cretaceous succession (Cenomanian ~ Santonian) of the Staffhorst Shaft, Lower Saxony, northern Germany: integrated biostratigraphic, lithostratigraphic and downhole geophysical log data. Acta Geologica Polonica, 49 (3),175-213. Warszawa. The Cenomanian to Santonian succession of the Staffhorst shaft, ca. 50 km south of Bremen, because of its struc tural position in the northel11 Gelman Upper Cretaceous basin, is intermediate in character and fossil content between the pelagic sediments characterizing the Pompeckj Block in the north and the proximal sediments of the Lower Saxony Block in the south. The biostratigraphic subdivision of the shaft is based on inoceramids, echi noids, belemnites and foraminifera. -
Um-Maps---G.Pdf
Map Title Author/Publisher Date Scale Catalogued Case Drawer Folder Condition Series or I.D.# Notes Topography, towns, roads, political boundaries for parts of Gabon - Libreville Service Géographique de L'Armée 1935 1:1,000,000 N 35 10 G1-A F One sheet Cameroon, Gabon, all of Equatorial Guinea, Sao Tomé & Principe Gambia - Jinnak Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 1 Towns, roads, political boundaries for parts of Gambia Gambia - N'Dungu Kebbe Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 2 Towns, roads, political boundaries for parts of Gambia Gambia - No Kunda Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 4 Towns, roads, political boundaries for parts of Gambia Gambia - Farafenni Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 5 Towns, roads, political boundaries for parts of Gambia Gambia - Kau-Ur Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 6 Towns, roads, political boundaries for parts of Gambia Gambia - Bulgurk Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 6 A Towns, roads, political boundaries for parts of Gambia Gambia - Kudang Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 7 Towns, roads, political boundaries for parts of Gambia Gambia - Fass Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 7 A Towns, roads, political boundaries for parts of Gambia Gambia - Kuntaur Directorate of Colonial Surveys 1948 1:50,000 N 35 10 G1-B G Sheet 8 Towns, roads, political -

Kurzportraiturzportrait
Stand 30.06.2015 KKurzportraiturzportrait Geschäftsstelle Wagenfeld Zentrale Groß Lessen Zentrale Groß Lessen Zahlen Daten Geschäftsstelle Drebber Fakten Die Genossenschaft Ansprechpartner RWG Groß Lessen-Diepholz eG Geschäftsstelle Telefon (0 42 71) 85 0 Raiffeisen-Warengenossenschaft Groß Lessen Telefax (0 42 71) 85 40 27232 Sulingen - Groß Lessen Geschäftsführer: Carsten Leymann 85 30 oder 01 71 522 38 36 Telefon (0 42 71) 85 0 Stellv. Geschäftsführung Susanne Nackenhorst 85 31 Telefax (0 42 71) 85 40 Verkauf / Außendienst Wilfried Albers 85 14 Internet: www.rwg-grosslessen.raiffeisen.de oder 01 71 789 43 43 e-mail: [email protected] Ralf Wacker 85 13 oder 01 75 201 19 80 Auftragsannahme Frank Denker 85 10 Matthias Sandfort 85 11 Gründung: 10. Dezember 1917 Disposition Mischfutter Henry Emker 85 24 Rohwaren Ein- u. Verkauf Karsten Markus 85 22 Mitglieder: 558 mit 825 Geschäftsanteilen Pflanzenschutzmittel/ Saatgut Klaus Höper 85 33 Geschäftsführer: Carsten Leymann Rechnungswesen Land- Maike Hansing 85 21 wirtschaft Marlis Fehner 85 21 Heidrun Krohn 85 21 Hauptstelle und Buchhaltung Henrik Möhlmeyer 85 20 Verwaltung: Groß Lessen 155 Erzeugergutschriften Jens Feldmann 85 23 Mitarbeiter: 52 und 3 Auszubildende Abteilung Mineralöl Telefax (0 42 71) 85 41 Heizöl, Diesel, Tankstellen David Heuermann 85 15 Geschäftsstellen: Robert Lüllmann 85 25 Biogas-Anlagen, Motorenöle Carsten Linderkamp 85 36 Rechnungswesen Mineralöl Yvonne Meyer 85 34 Groß Lessen Drebber Wagenfeld Bianca Rohlfing 85 34 Tel. (0 42 71) 85 0 Tel. (0 54 45) 997 90 Tel. (0 54 44) 980 060 Geschäftsstelle Telefon (0 54 44) 980 060 Lose- und Tütenumschlag, Wagenfeld Telefax (0 54 44) 980 06 19 Getreide, Raps, Düngemittel, Verkauf/Disposition/ Stefan Breywisch 980 06 10 Diesel, Heizöl, Biodiesel RME Düngermischanlage/ Mark Katt 980 06 11 Speise-, Industrie- und Fabrikkartoffeln, Raiffeisenmarkt Mark Ismer 980 06 13 Haus- und Gartenbedarf, landwirtschaftl. -

Jahresbericht 2016
Jahresbericht 2016 KREISSPARKASSE s GRAFSCHAFT DIEPHOLZ Sitz der Sparkasse und Geschäftsstellen Hauptstelle Diepholz, Sparkassenstraße 1 Telefon 05441 91-0 Telefax 05441 91-5199 [email protected] Internet www.kreissparkasse-diepholz.de Geschäftsstellen Barnstorf Neuenkirchen Barver (bis 31.12.2016) Rehden Diepholz -Süd Schwaförden (bis 31.12.2016) Drebber Siedenburg Drentwede (bis 31.12.2016) Ströhen Ehrenburg (bis 31.12.2016) Sudwalde Eydelstedt (bis 31.12.2016) Sulingen Kirchdorf Varrel Lembruch (bis 31.12.2016) Wagenfeld Lemförde Träger Landkreis Diepholz Diepholz, Niedersachsenstraße 2 Telefon 05441 976-0 Telefax 05441 976-1726 - 1 - Lagebericht 2016 ∗) Grundlagen der Sparkasse Die Sparkasse ist gemäß § 3 NSpG eine Anstalt des öffentlichen Rechts. Sie ist Mitglied des Sparkassenverbands Niedersachsen (SVN), Hannover, und über diesen dem Deut- schen Sparkassen- und Giroverband e.V. (DSGV), Berlin und Bonn, angeschlossen. Sie ist beim Amtsgericht Walsrode unter der Nummer HRA 100146 im Handelsregister eingetragen. Träger der Sparkasse ist der Landkreis Diepholz. Geschäftsgebiet der Sparkasse ist der südliche Teil des Landkreises Diepholz. Die Sparkasse ist Mitglied im bundesweiten institutsbezogenen Sicherungssystem der Sparkassen-Finanzgruppe. Die Sparkasse ist ein regionales Wirtschaftsunternehmen mit der Aufgabe, die geld- und kreditwirtschaftliche Versorgung der Bevölkerung und der Wirtschaft insbesonde- re im satzungsrechtlichen Geschäftsgebiet sicherzustellen. Daneben ist das soziale und kulturelle Engagement der -

Landkreis Diepholz
Amtsblatt für den Landkreis Diepholz Nr. 43/2020 vom 01.12.2020 Inhaltsverzeichnis A Bekanntmachungen des Landkreises Diepholz ................................................. 3 B Bekanntmachungen der Städte und Gemeinden ............................................... 3 Stadt Diepholz ................................................................................................................................ 3 Bekanntmachung der Genehmigung der 83. Änderung des Flächennutzungsplans der Stadt Diepholz „Sachlicher Teilflächennutzungsplan Windenergie“ ......................................................... 3 Stadt Twistringen ........................................................................................................................... 6 1. Nachtragshaushaltssatzung für das Haushaltsjahr 2020 ............................................................ 6 Samtgemeinde Bruchhausen-Vilsen ........................................................................................... 8 Haushaltssatzung des Abwasserzweckverbandes Thedinghausen / Bruchhausen-Vilsen für das Haushaltsjahr 2020 .......................................................................................................................... 8 98. Flächennutzungsplanänderung ............................................................................................... 10 Gemeinde Bruchhausen-Vilsen .................................................................................................. 11 Bauleitplanung der Gemeinde Bruchhausen-Vilsen - Bebauungsplan -

Erdöl Und Erdgas in Der Bundesrepublik Deutschland 2001
Erdöl und Erdgas in der Bundesrepublik Deutschland 2001 Söhlingen Z14 Frac 7 1500 m Frac 1 ca. 40 m 40 ca. m 00 ca. 1 Niedersächsisches Landesamt für Bodenforschung, Hannover Niedersächsisches Landesamt für Bodenforschung, Hannover Geological Survey of Lower Saxony Erdöl und Erdgas in der Bundesrepublik Deutschland 2001 MICHAEL PASTERNAK, MICHAEL KOSINOWSKI, JOACHIM LÖSCH, JÜRGEN MESSNER & ROBERT SEDLACEK Hannover 2002 Titelbild: Bei der Erschließung von Erdgasvorkommen aus extrem dichten Gesteinen wurde in Deutschland ein neuer Meilenstein in der Bohr- und Fractechnologie erreicht. Das Söhlingen- Konsortium, in dem die BEB Erdgas und Erdöl GmbH, die RWE-DEA AG und die Mobil Erd- gas-Erdöl GmbH (Operator) mit jeweils 30 Prozent, sowie die Wintershall AG mit 10 Prozent beteiligt sind, hat mit der Bohrung Söhlingen Z14 mittlerweile die dritte mehrfach gefracte Horizontalbohrung im dichten Dethlingen-Sandstein des Rotliegend abgeteuft. Mit der Söhlingen Z14 wurde dabei in einer Teufe von knapp 5.000 Metern erstmals eine Horizontalstrecke von 1.500 Metern erbohrt. Um eine wirtschaftliche Förderrate zu erzielen, wurden entlang der Horizontalstrecke im Abstand von jeweils 250 Metern sieben hydrauli- sche Fracs erzeugt. Nun kann das Erdgas großräumig in die mit keramischem Stützmittel gefüllten Risse im Gestein fließen, die wiederum das Gas weiter zum Bohrloch leiten. Trotz der sehr niedrigen Gesteinsdurchlässigkeit, die nur ein Hundertstel bis ein Tausendstel der Durchlässigkeit konventioneller Lagerstätten beträgt, können nun bis zu 25.000 Kubikmeter Erdgas pro Stunde aus dieser Bohrung gefördert werden. Die Anwendung innovativer Tech- nologien ist dabei der Schlüssel zum wirtschaftlichen Erfolg. Mit derartig neuen Technologien sollen künftig die bisher wirtschaftlich nicht förderbaren Erdgasressourcen aus dichten Lagerstätten in Deutschland entwickelt werden. -

Der Hannoveraner Eng
12|2020 DER HANNOVERANER No. 12 | December 2020 | Breeding Stallion of the Year: Forsyth FRH Licensing Changes take effect Sport Two Grand Prix-Winners Changes take effect A multitude of innovations were planned for this year’s licensing. Some had been in the planning and were a logical development; most, however, were a result of the restrictions due to the Covid-19 pandemic. All changes led to positive results. The only thing missing were the spectators and with them the atmosphere, the special morale and the exchange of ideas. By Ulrich Hahne or the first time, two discipline-related licen- na were increased. The degree of difficulty was not Fsing committees evaluated the stallions. This as high as in the second free-jumping session in had already been initiated in the spring. The most the large arena though. We forewent the lunging changes arose for the jumping stallions. All young of the jumping stallions. Separating the licensing stallions with jumping pedigrees came to Verden committee in two discipline-related committees for the pre-selections. It saved the youngsters an was the right step. Both committees discussed the additional trip for photos and video clips. On the horses while standing near them. The different ap- actual licensing, the demands for the previously proaches of the committee members became clear called “free-jumping test” in the old warm-up are- and a common thread was apparent. 2 Der Hannoveraner 12|2020 Only a few spectators were able to It was not originally planned to completely separa- and once as the dam’s sire), via Diarado and Dia- witness the spectacular perfor- te the disciplines during the licensing days. -
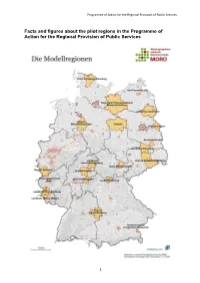
Facts and Figures About the Pilot Regions in the Programme of Action for the Regional Provision of Public Services
Programme of Action for the Regional Provision of Public Services Facts and figures about the pilot regions in the Programme of Action for the Regional Provision of Public Services 1 Programme of Action for the Regional Provision of Public Services District of Schleswig-Flensburg Composition of the region: 4 towns, 130 district municipalities grouped into 13 authorities for administrative purposes Data Area: 2,071 km² Population: 198,250 Population density: 95 inhabitants/km 2 Main areas to be addressed: Senior citizens and nursing care/people with disabilities Labour market and basic security benefits Child and family friendliness Culture and education Transport infrastructure (cross-cutting issue) Peenetal/Loitz Authority Composition of the region: Town of Loitz (lower-order centre), municipalities of Görmin, Sassen-Trantow, Düvier with a total of 31 communities; in the District of Western Pomerania-Greifswald Data Area: 170 km² Population: 6,700 Population density: 39 inhabitants/km 2 Main areas to be addressed: Living in harmony with nature Learning in harmony with nature Working in harmony with nature Resting in harmony with nature The infrastructure areas to be addressed will be specified at the launch of the pilot project. Western Mecklenburg Regional Planning Association Composition of the region: Districts of Northwestern Mecklenburg, Ludwigslust-Parchim, Urban District of Schwerin, 26 towns and 232 rural municipalities; one higher-order centre (Schwerin), five middle-order centres Data Area: 6,999 km² Population: 481,000 Population -

Commission Decision of 13 October 2006 Amending Decision 2005/393/EC As Regards the Conditions Applicable to Movements from Or T
Commission Decision of 13 October 2006 amending Decision 2005/393/EC as regards the conditions... 1 ANNEX Document Generated: 2021-03-26 Changes to legislation: There are currently no known outstanding effects for the Commission Decision of 13 October 2006 amending Decision 2005/393/EC as regards the conditions applicable to movements from or through restricted zones in relation to bluetongue (notified under document number C(2006) 4813) (Text with EEA relevance) (2006/693/EC), ANNEX. (See end of Document for details) ANNEX I. Annex I to Decision 2005/393/EC is amended as follows: 1. The list of restricted zones in Zone F (serotype 8) which relates to Germany is replaced by the following: Germany: Hessen Gesamtes Landesgebiet Niedersachsen — Im Landkeis Ammerland: Apen, Edewecht, Westerstede, Bad Zwischenahn — Im Landkreis Aurich: Krummhörn, Hinte, Ihlow — Landkreis Cloppenburg — Im Landkreis Diepholz: Stemshorn, Quernheim, Brockum, Marl, Hüde, Lembruch, Diepholz, Wetschen, Rehden, Hemsloh, Wagenfeld, Bahrenborstel, Kirchdorf, Varrel, Barver, Drebber, Dickel, Freistatt, Wehrlbleck, Barenburg, Maasen, Borstel, Sulingen, Eydelstedt, Barnstorf, Drentwede, Ehrenburg, Scholen, Schwaförden, Mellinghausen, Siedenburg, Staffhorst, Asendorf, Engeln, Affinghausen, Sudwalde, Neuenkirchen, Twistringen, Bassum, Lemförde — Stadt Emden — Landkreis Emsland — Im Landkreis Göttingen: Staufenberg, Hannoversch-Münden, Bühren, Scheden, Jühnde, Friedland, Gleichen, Rosdorf, Niemetal, Dransfeld, Landolfshausen, Waake, Ebergötzen, Wollbrandshausen, Krebeck, Bovenden, -

Windenergieanlagen Aktualisiert August 2019 Standortkonzept Windenergieanlagen 2018 – Stadt Sulingen Seite 2 Von 103
Stadt Sulingen Standortkonzept zur Steuerung von Windenergieanlagen Aktualisiert August 2019 Standortkonzept Windenergieanlagen 2018 – Stadt Sulingen Seite 2 von 103 Im Auftrag: Plan und Recht GmbH Prof.-Dr. jur. Gerd Schmidt-Eichstaedt Oderberger Straße 40 10435 Berlin Inhalt Inhalt 1 Anlass und Aufgabenstellung ................................................................................................. 5 2 Rechtliche Zulässigkeit von Windenergieanlagen (WEA) .................................................... 5 3 Rahmenbedingungen für das Standortkonzept .................................................................... 7 4 Aktuelle Situation in Sulingen – Bestand an WEA .............................................................. 7 5 Ziele und Vorgehensweise .................................................................................................... 12 5.1 Ziele ............................................................................................................................................................................ 12 5.2 Vorgehensweise ...................................................................................................................................................... 12 6 Festlegung der harten und weichen Tabukriterien als Ausschlusskriterien für WEA- Standorte ............................................................................................................................... 14 6.1 Tabukriterien nach dem RROP 2016 des Landkreises Diepholz ................................................................