Notes and News
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guia Para Observação Das Aves Do Parque Nacional De Brasília
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/234145690 Guia para observação das aves do Parque Nacional de Brasília Book · January 2011 CITATIONS READS 0 629 4 authors, including: Mieko Kanegae Fernando Lima Favaro Federal University of Rio de Janeiro Instituto Chico Mendes de Conservação da Bi… 7 PUBLICATIONS 74 CITATIONS 17 PUBLICATIONS 69 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Fernando Lima Favaro on 28 May 2014. The user has requested enhancement of the downloaded file. Brasília - 2011 GUIA PARA OBSERVAÇÃO DAS AVES DO PARQUE NACIONAL DE BRASÍLIA Aílton C. de Oliveira Mieko Ferreira Kanegae Marina Faria do Amaral Fernando de Lima Favaro Fotografia de Aves Marcelo Pontes Monteiro Nélio dos Santos Paulo André Lima Borges Brasília, 2011 GUIA PARA OBSERVAÇÃO DAS AVES DO APRESENTAÇÃO PARQUE NACIONAL DE BRASÍLIA É com grande satisfação que apresento o Guia para Observação REPÚblica FEDERATiva DO BRASIL das Aves do Parque Nacional de Brasília, o qual representa um importante instrumento auxiliar para os observadores de aves que frequentam ou que Presidente frequentarão o Parque, para fins de lazer (birdwatching), pesquisas científicas, Dilma Roussef treinamentos ou em atividades de educação ambiental. Este é mais um resultado do trabalho do Centro Nacional de Pesquisa e Vice-Presidente Conservação de Aves Silvestres - CEMAVE, unidade descentralizada do Instituto Michel Temer Chico Mendes de Conservação da Biodiversidade (ICMBio) e vinculada à Diretoria de Conservação da Biodiversidade. O Centro tem como missão Ministério do Meio Ambiente - MMA subsidiar a conservação das aves brasileiras e dos ambientes dos quais elas Izabella Mônica Vieira Teixeira dependem. -

ABSTRACT BOOK Listed Alphabetically by Last Name Of
ABSTRACT BOOK Listed alphabetically by last name of presenting author AOS 2019 Meeting 24-28 June 2019 ORAL PRESENTATIONS Variability in the Use of Acoustic Space Between propensity, renesting intervals, and renest reproductive Two Tropical Forest Bird Communities success of Piping Plovers (Charadrius melodus) by fol- lowing 1,922 nests and 1,785 unique breeding adults Patrick J Hart, Kristina L Paxton, Grace Tredinnick from 2014 2016 in North and South Dakota, USA. The apparent renesting rate was 20%. Renesting propen- When acoustic signals sent from individuals overlap sity declined if reproductive attempts failed during the in frequency or time, acoustic interference and signal brood-rearing stage, nests were depredated, reproduc- masking occurs, which may reduce the receiver’s abil- tive failure occurred later in the breeding season, or ity to discriminate information from the signal. Under individuals had previously renested that year. Addi- the acoustic niche hypothesis (ANH), acoustic space is tionally, plovers were less likely to renest on reservoirs a resource that organisms may compete for, and sig- compared to other habitats. Renesting intervals de- naling behavior has evolved to minimize overlap with clined when individuals had not already renested, were heterospecific calling individuals. Because tropical after second-year adults without prior breeding experi- wet forests have such high bird species diversity and ence, and moved short distances between nest attempts. abundance, and thus high potential for competition for Renesting intervals also decreased if the attempt failed acoustic niche space, they are good places to examine later in the season. Lastly, overall reproductive success the way acoustic space is partitioned. -

A Study of the Rufous-Fronted Thornbird and Associated Birds
A STUDY OF THE RUFOUS-FRONTED THORNBIRD AND ASSOCIATED BIRDS ALEXANDER F. SKUTCH ANGING from isolated trees in northern Venezuela are compact masses of H interlaced twigs so large and conspicuous that they can hardly escape the attention even of the traveler speeding along the excellent highways of that country. From a Venezuelan companion he may learn that the oblong masses of sticks are nests of the guaiti; but without patient watching he is not likely to see the retiring, wren-like builder of these incongruously large structures. The guaiti bears the English book-name of Rufous-fronted Thorn- bird (Phacellodomus rufifrons) and is a member of a large Neotropical family, the Furnariidae or ovenbirds, second to no other avian family in the world in the diversity of its nidification. The brief accounts that I had read of this remarkable bird, suggesting unusual social habits, whetted my desire to learn more about it. A grant from the Frank M. Chapman Memorial Fund of the American Museum of Natural History provided financial support for an extended visit to Venezuela. Cor- respondence with Paul Schwartz gave hope that we could find a suitable place to live while studying thornbirds. He met us at Maiquetia Airport soon after midnight on 12 March 1966 and, after showing us over the Parque National “Henri Pittier” (Ranch0 Grande), took us to “La Araguata.” This large cattle farm, belonging to the bird artist Walter Arp and his wife Elena, is situated, at an elevation around 1,400 feet, in a basin among low mountains near Pirapira, about 20 miles south of Valencia in the state of Carabobo. -

On the Origin and Evolution of Nest Building by Passerine Birds’
T H E C 0 N D 0 R r : : ,‘ “; i‘ . .. \ :i A JOURNAL OF AVIAN BIOLOGY ,I : Volume 99 Number 2 ’ I _ pg$$ij ,- The Condor 99~253-270 D The Cooper Ornithological Society 1997 ON THE ORIGIN AND EVOLUTION OF NEST BUILDING BY PASSERINE BIRDS’ NICHOLAS E. COLLIAS Departmentof Biology, Universityof California, Los Angeles, CA 90024-1606 Abstract. The object of this review is to relate nest-buildingbehavior to the origin and early evolution of passerinebirds (Order Passeriformes).I present evidence for the hypoth- esis that the combinationof small body size and the ability to place a constructednest where the bird chooses,helped make possiblea vast amountof adaptiveradiation. A great diversity of potential habitats especially accessibleto small birds was created in the late Tertiary by global climatic changes and by the continuing great evolutionary expansion of flowering plants and insects.Cavity or hole nests(in ground or tree), open-cupnests (outside of holes), and domed nests (with a constructedroof) were all present very early in evolution of the Passeriformes,as indicated by the presenceof all three of these basic nest types among the most primitive families of living passerinebirds. Secondary specializationsof these basic nest types are illustratedin the largest and most successfulfamilies of suboscinebirds. Nest site and nest form and structureoften help characterizethe genus, as is exemplified in the suboscinesby the ovenbirds(Furnariidae), a large family that builds among the most diverse nests of any family of birds. The domed nest is much more common among passerinesthan in non-passerines,and it is especially frequent among the very smallestpasserine birds the world over. -
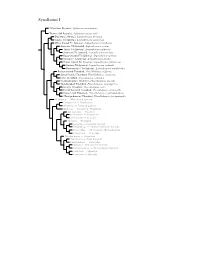
Synallaxini Species Tree
Synallaxini I ?Masafuera Rayadito, Aphrastura masafuerae Thorn-tailed Rayadito, Aphrastura spinicauda Des Murs’s Wiretail, Leptasthenura desmurii Tawny Tit-Spinetail, Leptasthenura yanacensis White-browed Tit-Spinetail, Leptasthenura xenothorax Araucaria Tit-Spinetail, Leptasthenura setaria Tufted Tit-Spinetail, Leptasthenura platensis Striolated Tit-Spinetail, Leptasthenura striolata Rusty-crowned Tit-Spinetail, Leptasthenura pileata Streaked Tit-Spinetail, Leptasthenura striata Brown-capped Tit-Spinetail, Leptasthenura fuliginiceps Andean Tit-Spinetail, Leptasthenura andicola Plain-mantled Tit-Spinetail, Leptasthenura aegithaloides Rufous-fronted Thornbird, Phacellodomus rufifrons Streak-fronted Thornbird, Phacellodomus striaticeps Little Thornbird, Phacellodomus sibilatrix Chestnut-backed Thornbird, Phacellodomus dorsalis Spot-breasted Thornbird, Phacellodomus maculipectus Greater Thornbird, Phacellodomus ruber Freckle-breasted Thornbird, Phacellodomus striaticollis Orange-eyed Thornbird, Phacellodomus erythrophthalmus ?Orange-breasted Thornbird, Phacellodomus ferrugineigula Hellmayrea — White-tailed Spinetail Coryphistera — Brushrunner Anumbius — Firewood-gatherer Asthenes — Canasteros, Thistletails Acrobatornis — Graveteiro Metopothrix — Plushcrown Xenerpestes — Graytails Siptornis — Prickletail Roraimia — Roraiman Barbtail Thripophaga — Speckled Spinetail, Softtails Limnoctites — ST Spinetail, SB Reedhaunter Cranioleuca — Spinetails Pseudasthenes — Canasteros Spartonoica — Wren-Spinetail Pseudoseisura — Cacholotes Mazaria – White-bellied -

Northern Argentina Tour Report 2016
The enigmatic Diademed Sandpiper-Plover in a remote valley was the bird of the trip (Mark Pearman) NORTHERN ARGENTINA 21 OCTOBER – 12 NOVEMBER 2016 TOUR REPORT LEADER: MARK PEARMAN Northern Argentina 2016 was another hugely successful chapter in a long line of Birdquest tours to this region with some 524 species seen although, importantly, more speciality diamond birds were seen than on all previous tours. Highlights in the north-west included Huayco Tinamou, Puna Tinamou, Diademed Sandpiper-Plover, Black-and-chestnut Eagle, Red-faced Guan, Black-legged Seriema, Wedge-tailed Hilstar, Slender-tailed Woodstar, Black-banded Owl, Lyre-tailed Nightjar, Black-bodied Woodpecker, White-throated Antpitta, Zimmer’s Tapaculo, Scribble-tailed Canastero, Rufous-throated Dipper, Red-backed Sierra Finch, Tucuman Mountain Finch, Short-tailed Finch, Rufous-bellied Mountain Tanager and a clean sweep on all the available endemcs. The north-east produced such highly sought-after species as Black-fronted Piping- Guan, Long-trained Nightjar, Vinaceous-breasted Amazon, Spotted Bamboowren, Canebrake Groundcreeper, Black-and-white Monjita, Strange-tailed Tyrant, Ochre-breasted Pipit, Chestnut, Rufous-rumped, Marsh and Ibera Seedeaters and Yellow Cardinal. We also saw twenty-fve species of mammal, among which Greater 1 BirdQuest Tour Report: Northern Argentina 2016 www.birdquest-tours.com Naked-tailed Armadillo stole the top slot. As usual, our itinerary covered a journey of 6000 km during which we familiarised ourselves with each of the highly varied ecosystems from Yungas cloud forest, monte and badland cactus deserts, high puna and altiplano, dry and humid chaco, the Iberá marsh sytem (Argentina’s secret pantanal) and fnally a week of rainforest birding in Misiones culminating at the mind-blowing Iguazú falls. -

Association with Trypanosoma Cruzi, Different Habitats
Revista da Sociedade Brasileira de Medicina Tropical 48(5):532-538, Sep-Oct, 2015 Major Article http://dx.doi.org/10.1590/0037-8682-0184-2015 Triatominae (Hemiptera, Reduviidae) in the Pantanal region: association with Trypanosoma cruzi, diff erent habitats and vertebrate hosts Filipe Martins Santos[1], Ana Maria Jansen[2], Guilherme de Miranda Mourão[3], José Jurberg[4], Alessandro Pacheco Nunes[5] and Heitor Miraglia Herrera[1] [1]. Laboratório de Parasitologia Animal, Universidade Católica Dom Bosco, Campo Grande, Mato Grosso do Sul, Brazil. [2]. Laboratório de Biologia de Tripanossomatídeos, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil. [3]. Laboratório de Vida Selvagem, Centro de Pesquisa Agropecuária do Pantanal/Embrapa-Pantanal, Corumbá, Mato Grosso do Sul, Brazil. [4]. Laboratório Nacional e Internacional de Referência em Taxonomia de Triatomíneos, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil. [5]. Programa de Pós-Graduação em Ecologia e Conservação, Universidade Federal de Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brazil. ABSTRACT Introduction: The transmission cycle of Trypanosoma cruzi in the Brazilian Pantanal region has been studied during the last decade. Although considerable knowledge is available regarding the mammalian hosts infected by T. cruzi in this wetland, no studies have investigated its vectors in this region. This study aimed to investigate the presence of sylvatic triatomine species in different habitats of the Brazilian Pantanal region and to correlate their presence with the occurrences of vertebrate hosts and T. cruzi infection. Methods: The fi eldwork involved passive search by using light traps and Noireau traps and active search by visual inspection. -

A New Genus and Species of Furnariid (Aves: Furnariidae) from the Cocoa-Growing Region of Southeastern Bahia, Brazil
THEWILSONBULLETIN A QUARTERLY MAGAZINE OF ORNITHOLOGY Published by the Wilson Ornithological Society VOL. 108, No. 3 SEPTEMBER 1996 PAGES 397-606 Wilson Bull., 108(3), 1996, pp. 397-433 A NEW GENUS AND SPECIES OF FURNARIID (AVES: FURNARIIDAE) FROM THE COCOA-GROWING REGION OF SOUTHEASTERN BAHIA, BRAZIL Jose FERNANDO PACHECO,’ BRET M. WHITNEY,‘J AND LUIZ P. GONZAGA’ ABSTRACT.-we here describe Acrobatomis fonsecai, a new genus and species in the Furnariidae, from the Atlantic Forest of southeastern Bahia, Brazil. Among the outstanding features of this small, arboreal form are: black-and-gray definitive plumage lacking any rufous: juvenal plumage markedly different from adult; stout, bright-pink legs and feet; and its acrobatic foraging behavior involving almost constant inverted hangs on foliage and scansorial creeping along the undersides of canopy limbs. Analysis of morphology, vocal- izations, and behavior suggest to us a phylogenetic position close to Asfhenes and Crani- oleuca; in some respects, it appears close to the equally obscure Xenerpesres and Meto- pothrix. New data on the morphology, vocalizations, and behavior of several furuariids possibly related to Acrobatornis are presented in the context of intrafamilial relationships. We theorize that Acrobatornis could have colonized its current range during an ancient period of continental semiaridity that promoted the expansion of stick-nesting prototypes from a southern, Chaco-PatagonianE’antanal center, and today represents a relict that sur- vived by adapting to build its stick-nest in the relatively dry, open, canopy of leguminaceous trees of the contemporary humid forest in southeastern Bahia. Another theory of origin places emphasis on the fact that the closest relatives of practically all (if not all) other birds syntopic with Acrobatomis are of primarily Amazonian distribution. -

Museum Quarterly LSU Museum of Natural Science
Museum Quarterly LSU Museum of Natural Science February 2010 Volume 28, Issue 1 Letter from the Director... Museum of 2009: A Good Year Natural Science Although 2009 was a grim year for the economy, it was a Director and great year for the Museum. Let me count the ways. Curators Awards and honors abounded. Curator of Mammals Mark Hafner was voted a Fellow of the AAAS, and he received a Frederick H. Sheldon “Dean’s Commendation for Excellence in Teaching.” Curator Director, George H. Lowery, of Birds received the LSU Foundation Distin- Jr., Professor, and Van Remsen Curator of Genetic guished Faculty Teaching Award. Graduate student Gustavo Resources Bravo received an Outstanding Student Presentation Award at the annual meeting of the American Ornithologists’ Union. Christopher C. Austin (LSU students have won this award four years in a row.) Curator of Curator of Education Sophie Warny was elected to the board of the American Association Herpetology of Stratigraphic Palynologists. Grad student James Maley received the T. Vinton Holmes Award and HC Lim received the Outstanding International Student Award from the LSU Robb T. Department of Biological Sciences. Grad student César Sanchez received the Virginia L. Brumfield Mouw Ornithology Award and the Outstanding Graduate Student Award from Curator of Luciano Naka Genetic Resources the Museum of Natural Science. Prosanta Chakrabarty Six students graduated. Travis Atwood, M.S. Geology & Geophysics; Amber Curator of Fishes Gates, M.S. Biological Sciences; Curt Burney, Ph.D. Biological Sciences; Brian O’Shea, Ph.D. Biological Sciences; Thomas Valqui, Ph.D. Biological Sciences; and Michael Wil- Mark S. -

Description of an Amazing Nest of Caatinga Cachalote
doi: 10.5216/rpt.v47i2.54214 BRIEF REPORT DESCRIPTION OF AN AMAZING NEST OF CAATINGA CACHALOTE Pseudoseisura cristata INFESTED BY Psammolestes tertius IN TAUÁ, STATE OF CEARÁ, NortHEASTERN BRAZIL Liléia Gonçalves Diotaiuti1, Claudia Mendonça Bezerra2, Cyro José Soares3, Lilian Mariana Costa4 and Silvia Ermelinda Barbosa1 ABSTRACT Psammolestes tertius Lent & Jurberg 1965 is a triatomine species part of the tribe Rhodniini. It is only wild and found in bird nests, mostly of the Furnariidae family. This work describes the amazing infestation of a nest of Pseudoseisura cristata in the municipality of Taua, Ceará. The nest harbored a colony with about 40 specimens of P. tertius in all evolutionary stages, all negative for trypanosomatids. A rodent of the species Wiedomys pyrrhorhinos was seen running away from the nest. The materials used to build the nest included the typical natural wigs, and various anthropogenic materials such as razor blades, shaver, fork, sunglasses, metal fragments, fragments of plastic objects, wire, barbed wire, plastic sandal straps, paper, plastic lid, pieces of plastic bags, gas cylinder label, large feathers and the exuviae of a snake. KEY WORDS: Psammolestes tertius; triatomine; Pseudoseisura cristata; birds Psammolestes tertius Lent & Jurberg, 1965 is a triatomine species that is part of the tribe Rhodniini. It is only wild and found in bird nests, mostly of the Furnariidae family (Lent & Wygozinsky, 1979; Di Iorio & Turienzo, 2009). This species was reported colonizing nests of the following furnariids: Rufous-fronted Thornbird Phacellodomus rufifrons (Wied-Neuwied, 1821), Greater Thornbird Phacellodomus ruber (Vieillot, 1817), Firewood-gatherer Anumbius annumbi (Vieillot, 1817), and Caatinga Cachalote Pseudoseisura cristata (Spix, 1824) (Di Iorio & Turienzo, 2009). -

Note to Users
NOTE TO USERS This reproduction is the best copy available. UMI* EXPLORING THE EFFICACY, UTILITY, AND LIMITATIONS OF DNA BARCODING WITHIN THE CLASS AVES A Thesis Presented to The Faculty of Graduate Studies of The University of Guelph by KEVIN CHARLES ROBERT KERR In partial fulfilment of requirements for the degree of Doctor of Philosophy April, 2010 © Kevin C. R. Kerr, 2010 Library and Archives Bibliotheque et 1*1 Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A 0N4 Ottawa ON K1A 0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-64533-8 Our file Notre reference ISBN: 978-0-494-64533-8 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distribute and sell theses monde, a des fins commerciales ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in this et des droits moraux qui protege cette these. Ni thesis. Neither the thesis nor la these ni des extraits substantiels de celle-ci substantial extracts from it may be ne doivent etre imprimes ou autrement printed or otherwise reproduced reproduits sans son autorisation. -

BRAZIL: Atlantic Forest Highlights – July 2019
Tropical Birding - Trip Report BRAZIL: Atlantic Forest Highlights – July 2019 A Tropical Birding Set Departure BIRDING TOUR (www.shorturl.at/cpq89) Brazil: Atlantic Forest Highlights 13-20 July, 2019 Report and photos by ANDRES VASQUEZ N., the guide for this tour One of the great things about this tour is the extremely pleasant combination of “good-old-fashion” forest birding with incredibly active and diverse feeders. This Festive Coquette (picture above) is one of the birds that we basically manage to enjoy only thanks to the feeders at Folhia Seca near Ubatuba; this incredibly ornate species is otherwise hard to pick up in the canopy of tall tropical forest due to its tiny size and fairly non-contrasting colors. www.tropicalbirding.com +1-409-515-9110 [email protected] p.1 Tropical Birding - Trip Report BRAZIL: Atlantic Forest Highlights – July 2019 INTRODUCTION: The Atlantic Forest of Southeast Brazil is a very well-known Endemic Bird Area (EBA) of the World thanks not only to its richness, in terms of endemic species but also thanks to the fact that most of those endemic birds are absolutely spectacular. This makes that this region of the globe is in the bucket list of any birdwatcher that wants to fill his/her list with A TON of brightly colorful endemic birds. Now, this area is vast and in order to cover it all we have to drive considerably long distances in over three weeks of birding (which we do in our comprehensive SE Brazil tour, see here: www.shorturl.at/isuCN) BUT FORTUNATELY for the birders that do not have all this long time off we created this shorter trip, The Atlantic Forest Highlights tour, which very well represents “in a nutshell” the greatness of this region since it includes most of the very nicest-looking birds of the whole EBA.