Risk-Based Criteria to Support Validation of Detection Methods for Scdrinking Water and Air IEN
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

Carbamate Pesticides Aldicarb Aldicarb Sulfoxide Aldicarb Sulfone
Connecticut General Statutes Sec 19a-29a requires the Commissioner of Public Health to annually publish a list setting forth all analytes and matrices for which certification for testing is required. Connecticut ELCP Drinking Water Analytes Revised 05/31/2018 Microbiology Total Coliforms Fecal Coliforms/ E. Coli Carbamate Pesticides Legionella Aldicarb Cryptosporidium Aldicarb Sulfoxide Giardia Aldicarb Sulfone Carbaryl Physicals Carbofuran Turbidity 3-Hydroxycarbofuran pH Methomyl Conductivity Oxamyl (Vydate) Minerals Chlorinated Herbicides Alkalinity, as CaCO3 2,4-D Bromide Dalapon Chloride Dicamba Chlorine, free residual Dinoseb Chlorine, total residual Endothall Fluoride Picloram Hardness, Calcium as Pentachlorophenol CaCO3 Hardness, Total as CaCO3 Silica Chlorinated Pesticides/PCB's Sulfate Aldrin Chlordane (Technical) Nutrients Dieldrin Endrin Ammonia Heptachlor Nitrate Heptachlor Epoxide Nitrite Lindane (gamma-BHC) o-Phosphate Metolachlor Total Phosphorus Methoxychlor PCB's (individual aroclors) Note 1 PCB's (as decachlorobiphenyl) Note 1 Demands Toxaphene TOC Nitrogen-Phosphorus Compounds Alachlor Metals Atrazine Aluminum Butachlor Antimony Diquat Arsenic Glyphosate Barium Metribuzin Beryllium Paraquat Boron Propachlor Cadmium Simazine Calcium Chromium Copper SVOC's Iron Benzo(a)pyrene Lead bis-(2-ethylhexyl)phthalate Magnesium bis-(ethylhexyl)adipate Manganese Hexachlorobenzene Mercury Hexachlorocyclopentadiene Molybdenum Nickel Potassium Miscellaneous Organics Selenium Dibromochloropropane (DBCP) Silver Ethylene Dibromide (EDB) -

Warfare Agents for Modeling Airborne Dispersion in and Around Buildings
LBNL-45475 ERNEST ORLANDO LAWRENCE BERKELEY NATIn NAL LABORATORY Databaseof Physical,Chemicaland ToxicologicalPropertiesof Chemical and Biological(CB)WarfitreAgentsfor ModelingAirborneDispersionIn and AroundBuildings TracyThatcher,RichSextro,andDonErmak Environmental Energy Technologies Division DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor The Regents of the University of Catifomia, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of anY information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommend at i on, or favoring by the United States Government or any agency thereof, or The Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof, or The Regents of the University of California. Ernest Orlando Lawrence Berkeley National Laboratory is an equal opportunity employer. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced -
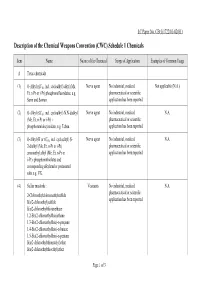
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard
Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude To cite this version: Juan-Carlos Lizardo-Huerta, Baptiste Sirjean, Laurent Verdier, René Fournet, Pierre-Alexandre Glaude. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas. Journal of Physical Chemistry A, American Chemical Society, 2017, 121 (17), pp.3254-3262. 10.1021/acs.jpca.7b01238. hal-01708219 HAL Id: hal-01708219 https://hal.archives-ouvertes.fr/hal-01708219 Submitted on 13 Feb 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas Juan-Carlos Lizardo-Huerta†, Baptiste Sirjean†, Laurent Verdier‡, René Fournet†, Pierre-Alexandre Glaude†,* †Laboratoire Réactions et Génie des Procédés, CNRS, Université de Lorraine, 1 rue Grandville BP 20451 54001 Nancy Cedex, France ‡DGA Maîtrise NRBC, Site du Bouchet, 5 rue Lavoisier, BP n°3, 91710 Vert le Petit, France *corresponding author: [email protected] Abstract The destruction of stockpiles or unexploded ammunitions of nitrogen mustard (tris (2- chloroethyl) amine, HN-3) requires the development of safe processes. -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention. -

Nerve Agent - Lntellipedia Page 1 Of9 Doc ID : 6637155 (U) Nerve Agent
This document is made available through the declassification efforts and research of John Greenewald, Jr., creator of: The Black Vault The Black Vault is the largest online Freedom of Information Act (FOIA) document clearinghouse in the world. The research efforts here are responsible for the declassification of MILLIONS of pages released by the U.S. Government & Military. Discover the Truth at: http://www.theblackvault.com Nerve Agent - lntellipedia Page 1 of9 Doc ID : 6637155 (U) Nerve Agent UNCLASSIFIED From lntellipedia Nerve Agents (also known as nerve gases, though these chemicals are liquid at room temperature) are a class of phosphorus-containing organic chemicals (organophosphates) that disrupt the mechanism by which nerves transfer messages to organs. The disruption is caused by blocking acetylcholinesterase, an enzyme that normally relaxes the activity of acetylcholine, a neurotransmitter. ...--------- --- -·---- - --- -·-- --- --- Contents • 1 Overview • 2 Biological Effects • 2.1 Mechanism of Action • 2.2 Antidotes • 3 Classes • 3.1 G-Series • 3.2 V-Series • 3.3 Novichok Agents • 3.4 Insecticides • 4 History • 4.1 The Discovery ofNerve Agents • 4.2 The Nazi Mass Production ofTabun • 4.3 Nerve Agents in Nazi Germany • 4.4 The Secret Gets Out • 4.5 Since World War II • 4.6 Ocean Disposal of Chemical Weapons • 5 Popular Culture • 6 References and External Links --------------- ----·-- - Overview As chemical weapons, they are classified as weapons of mass destruction by the United Nations according to UN Resolution 687, and their production and stockpiling was outlawed by the Chemical Weapons Convention of 1993; the Chemical Weapons Convention officially took effect on April 291997. Poisoning by a nerve agent leads to contraction of pupils, profuse salivation, convulsions, involuntary urination and defecation, and eventual death by asphyxiation as control is lost over respiratory muscles. -

P-Listed Hazardous Wastes
P-Listed Hazardous Wastes The Environmental Protection Agency (EPA) has identified a number of chemicals on the EPA “P-list” that present an especially acute hazard when disposed of as hazardous waste. Because of their acute hazards, there are more stringent requirements when disposing of these wastes: ►Container size: When collecting p-listed chemicals as waste, the volume of the hazardous waste container must not exceed one quart (approximately one liter). ►Empty containers: Empty containers that held p-listed chemicals must also be disposed of as hazardous waste. They are not allowed to be washed or re-used. ►Contaminated materials: Disposable materials that become contaminated with p-listed chemicals (e.g. gloves, weighing boats, etc.) must also be disposed of as hazardous waste. Non-disposable materials must be “triple-rinsed”, or rinsed three times to remove the contamination. This rinsate must be collected as hazardous waste. Materials contaminated with p-listed chemicals may not be washed or re-used until they have been triple-rinsed. Remember: - Label the waste as hazardous waste. Most common p-listed wastes Just like all other hazardous wastes, p-listed Chemical CAS number wastes must be labeled with the words Acrolein 107–02–8 “hazardous waste”, the complete chemical Allyl alcohol 107–18–6 name, and the associated hazard Arsenic compounds Varies characteristics (e.g., ignitable, corrosive, Inorganic cyanide Varies toxic, or reactive). salts Carbon disulfide 75-15-0 - Use disposable materials whenever Cyanogen and 460-19-5, 506-77-4 possible. Triple-rising non-disposable Cyanogen Chloride material generates a lot of waste, which can 2,4-Dinitrophenol 51–28–5 be difficult to dispose of safely. -

Fumigation for Rabbit Control
Fumigation for rabbit control Introduction: Fumigation is a valuable tool in successful rabbit management programs. It is an effective follow-up technique to poison baiting and warren ripping, and is a particularly useful control method in areas where other techniques cannot be used (eg poison baiting). Fumigation works by replacing the air in warrens with Figure 1: Phostoxin tablets and dry paper are pushed deep lethal gasses, which are in-turn inhaled by rabbits, into the warren entrance. Image: Invasive Animals CRC causing them to suffocate and die. There are two types of fumigation: Fumigants Chloropicrin: Chloropicrin is a colourless, toxic liquid • pressure fumigation - where the fumigant is generated that is currently used in Australia as an insecticide, soil outside the warren and forced into the warren under and warren fumigant, and rodenticide. Chloropicrin pressure, usually from a pump is classified as a dangerous poison (Schedule 7) as • diffusion (or static) fumigation – where tablets are placed it has a high potential to cause harm, even at low in active burrows (Figure 1) and the fumigant generated exposures. Chloropicrin has recently been placed under is allowed to passively diffuse through the warren (this is review by the Australian Pesticides and Veterinary the preferred method)1. Medicines Authority (APVMA) because of environmental, There are strict regulations around the use of fumigants human health and human safety concerns. It is not for rabbit control that reduce the risk of harm to recommended for use, even though it is still registered operators. For this reason, users may also require training in some states. Pressure fumigation using chloropicrin ® or accreditation before using approved fumigants. -

Combustion and Pyrolysis Kinetics of Chloropicrin J.-C
Combustion and Pyrolysis Kinetics of Chloropicrin J.-C. Lizardo-Huerta, B. Sirjean, L. Verdier, R. Fournet, Pierre-Alexandre Glaude To cite this version: J.-C. Lizardo-Huerta, B. Sirjean, L. Verdier, R. Fournet, Pierre-Alexandre Glaude. Combustion and Pyrolysis Kinetics of Chloropicrin. Journal of Physical Chemistry A, American Chemical Society, 2018, 122 (26), pp.5735 - 5741. 10.1021/acs.jpca.8b04007. hal-01921757 HAL Id: hal-01921757 https://hal.univ-lorraine.fr/hal-01921757 Submitted on 14 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Combustion and Pyrolysis Kinetics of Chloropicrin J.-C. Lizardo-Huerta1, B. Sirjean1, L. Verdier2, R. Fournet1, P.-A. Glaude1* 1 Laboratoire Réactions et Génie des Procédés, CNRS, Université de Lorraine 1 rue Grandville BP 20451 54001 Nancy Cedex, France 2 DGA Maîtrise NRBC, Site du Bouchet, 5 rue Lavoisier, BP n°3, 91710 Vert le Petit, France Corresponding author : Pierre-Alexandre Glaude Laboratoire Réactions et Génie des Procédés 1 rue Grandville BP 20451 54001 Nancy Cedex, France Email:[email protected] 1 Abstract Chloropicrin (CCl3NO2) is widely used in agriculture as a pesticide, weed-killer, fungicide or nematicide. -

Alcoholsalcohols
AlcoholsAlcohols Chapter 10 1 Structure of Alcohols • The functional group of an alcohol is H an -OH group bonded to an sp3 O 108.9° hybridized carbon. C H – Bond angles about the hydroxyl oxygen H H atom are approximately 109.5°. • Oxygen is sp3 hybridized. –Two sp3 hybrid orbitals form sigma bonds to a carbon and a hydrogen. – The remaining two sp3 hybrid orbitals each contain an unshared pair of electrons. 2 Nomenclature of Alcohols • IUPAC names – The parent chain is the longest chain that contains the OH group. – Number the parent chain to give the OH group the lowest possible number. – Change the suffix -etoe -ol.ol • Common names – Name the alkyl group bonded to oxygen followed by the word alcohol.alcohol 3 Nomenclature of Alcohols OH o o 1o OH 1 2 OH 1-Propanol 2-Propan ol 1-Bu tanol (Pro py l alco ho l) (Isoprop yl alcoh ol) (Bu tyl alcoh ol) OH 2o OH 1o OH 3o 2-Butanol 2-M eth yl-1-p ropan ol 2-M eth yl-2-p ropan ol (sec-Butyl alcohol) (Isobutyl alcohol) (tert -Butyl alcohol) 4 Nomenclature of Alcohols • Compounds containing more than one OH group are named diols, triols, etc. CH2 CH2 CH3 CHCH2 CH2 CHCH2 OH OH HO OH HO HO OH 1,2-Ethanediol 1,2-Propanediol 1,2,3-Propanetriol (Ethylene glycol) (Propylene glycol) (Glycerol, Glycerine) 5 Physical Properties • Alcohols are polar compounds. – They interact with themselves and with other polar compounds by dipole-dipole interactions. • Dipole-dipole interaction: The attraction between the positive end of one dipole and the negative end of another.