Antidiabetic Compounds from Hypoxis Colchicifolia and Terminalia Sericea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

117 La Familia Combretaceae En La Cuenca Del Río Balsas
Núm.19, pp.117-153, ISSN 1405-2768; México, 2005 LA FAMILIA COMBRETACEAE EN LA CUENCA DEL RÍO BALSAS, MÉXICO Erika Margarita Pagaza Calderón Rafael Fernández Nava Laboratorio de Fanerógamas, Departamento de Botánica, Escuela Nacional de Ciencias Biológicas, IPN Apartado Postal 17-564, México, DF, CP 11410, MÉXICO RESUMEN Dentro del área de estudio se reconoce la existencia de cinco géneros con ocho En el presente trabajo se realizó una revisión especies de la familia Combretaceae: Bucida taxonómica de la familia Combretaceae para wiginsiana, Combretum argenteum, C. el área de la cuenca del río Balsas; se decandrum, C. fruticosum, C. laxum, incluyen descripciones y claves Conocarpus erecta, Laguncularia racemosa, dicotómicas para la identificación de los y Terminalia catappa. El género Bucida es géneros y las especies que se distribuyen reportado por primera vez para el área de dentro de la zona de estudio. estudio. El proyecto se desarrolló mediante la ABSTRACT revisión de más de 200 ejemplares de los herbarios de las siguientes instituciones: In this paper we present a taxonomic Escuela Nacional de Ciencias Biológicas revision of the family Combretaceae for the (ENCB), Instituto de Biología de la UNAM Balsas River basin, Mexico. The study area (MEXU), Facultad de Ciencias de la UNAM covers part of 8 states of our country (State (FCME), Universidad Autónoma de of Mexico, Guerrero, Jalisco, Michoacan, Chapingo, Sección de Posgrado (CHAP); Morelos, Oaxaca, Puebla and Tlaxcala). Instituto Nacional de Investigaciones Forestales (INIF), Universidad Autónoma The present work includes descriptions and del Estado de Morelos (HUMO) y la dichotomous keys for identifying genera Universidad Autónoma Metropolitana and species distribuited on this area. -

Drivers of Woody Plant Form and Function in Relation to Water Acquisition and Use in Seasonal Forests
Drivers of woody plant form and function in relation to water acquisition and use in seasonal forests A Dissertation SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Christina Marie Smith IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Jennifer S. Powers, Advisor June 2019 © Christina Marie Smith, June 2019 All rights reserved. Acknowledgements First and foremost, I thank my advisor, Dr. Jennifer Powers, for her outstanding guidance, patience, and support throughout this whole process. Jennifer’s passion for understanding how tropical dry forests function is inspiring and motivated me to conduct the research in this dissertation. I will always be incredibly grateful for the countless steps Jennifer has taken to aid my development as a scientist and I could not have wished for a better advisor. I also thank, Dr. Tim Brodribb and Dr. Stefan Schnitzer, who at times served as my honorary advisors, for their advice and help. My present committee members Dr. Rebecca Montgomery, Dr. Jeannine Cavender-Bares, and Dr. Walid Sadok, and past members, Dr. David Moeller, and Dr. Peter Kennedy have provided support and helpful feedback over the years. I am thankful for many past and present members of the Powers’ lab. In particular, I think Dr. Leland Werden for all his support and advice throughout these years and for all the fun times we had while doing fieldwork. I am also very grateful to Laura Toro for all her help and friendship. It has also been a pleasure to get to know and collaborate with German Vargas, Dr. Naomi Schwartz, Dr. -

Check List the Journal Of
12 2 1859 the journal of biodiversity data 21 March 2016 Check List LISTS OF SPECIES Check List 12(2): 1859, 21 March 2016 doi: http://dx.doi.org/10.15560/12.2.1859 ISSN 1809-127X © 2016 Check List and Authors Checklist of the vascular flora of Reserva Biológica San Luis, Costa Rica José Esteban Jiménez1*, Pedro Juárez2 and Armando Díaz2 1 Programa Regional de Posgrado en Biología, Universidad de Costa Rica, Apdo. 11501−2060, San José, Costa Rica 2 Escuela de Ciencias Ambientales, Universidad Nacional de Costa Rica, Heredia, Costa Rica * Corresponding author. E-mail: [email protected] Abstract: The Reserva Biológica San Luis is a small vegetation. Located on the Pacific slope of the Cordillera protected area located on the Pacific side of the de Tilarán, the Reserva Biologica San Luis (RBSL) is a Cordillera de Tilarán, northwestern Costa Rica, with a small protected area that preserves a seasonal and forest transitioning between the basal and premontane transitional vegetation between the lowland tropical floras according to Holdridge’s Life Zones. An inventory wet forest and premontane wet forest (Holdridge 1967). of the vascular flora of the reserve was performed by This vegetation type is usually located between 500–800 collecting botanical samples during three years and m on the Pacific slope of Costa Rican mountain ranges, consulting the databases of the CR, INB, MO and USJ especially in the seasonal Central Pacific region, the herbaria. We report 130 families, 477 genera and 716 North Pacific region and in the Central Valley. species of native vascular plants. -

Evolution and Pollination of New Zealand Myosotis
EVOLUTION AND POLLINATION OF NEW ZEALAND MYOSOTIS (BORAGINACEAE) A thesis submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy in the University of Canterbury by Alastair W. Robertson University of Canterbury 1989 Myosoti australis var conspicua Fog Peak. Ca nterbury. Dedica ed to David Ll oyd, whose enthusi sm for his subject is an inspiration to us all. )' ,r _, TABLE OF CONTENTS Chapter Page Abstract An introduction to the systematics, biogeography and floral 2 biology of Myosotis in New Zealand. 2 Herkogamy, dichogamy and self-pollination in six species of Myosotis 38 3 Rates of pollen deposition and removal in M.colensoi and comparisons of 64 effective and nominal phase durations. 4 The effect of flower display size on visitation rates and reproductive 92 success in M.colensoi. 5 The relationships between floral display size, pollen carryover and 112 geitonogamy in M.colensoi. 6 Movement patterns of pollinators within plants of Myosotis colensoi 130 References 148 Acknowledgements 159 ABSTRACT The indigenous New Zealand species of Myosotis all belong to the southern section Exarrhena. The sister group to the southern species appears to be the discolor-group of eastern Africa and western Europe. It is postulated that the genus has an long history in New Zealand and is not the result of recent immigration from the northern hemisphere. In New Zealand, extensive speciation has occurred. Approximately 47 species are recognised. Radiation has proceeded in several different directions with respect to floral form. Several floral syndromes are recognised including tube-, funnel- and brush-blossoms. Some species have tended towards autogamy with a reduction in herkogamy, dichogamy, pollen:ovule ratio and petal size. -

Descriptive Part 145
BLUMEA 25 (1979) 141-223 Wood anatomy oftheCombretaceae G.J.C.M. van Vliet Hortus Botanicus, Plantage Middenlaan 2, Amsterdam, The Netherlands Summary The wood ofall of the Combretaceae is described in detail anatomy genera (Meiostemonexcepted) on 19 data the basis of 120 samples representing 90 species from genera. Additional from the literature are added. The structural variation of the vestured pits is described and classified. There aretwo main types, of which the distribution follows the subfamilyclassification. Consideringthe overall wood anatomy, the recognition of two subfamilies: Strephonematoideae (Strephonema only) and Combretoideae (all of its of remaining genera) can be supported. Strephonema stands out on account fibre-tracheids, type vesturing and parenchyma distribution pattern. Within Combretoideae, one group of genera (subtribe Combretinae sensuExell & Stace)stands out markedly on account of their radial vessels, a uniquefeature known in other and distinct size classes of vessel elements. The not to occur any plant group, two remaining genera, belongingto the tribe Laguncularieaeand subtribes Terminaliinae and Pteleopsidinae of tribe Combreteae show a wide overlap in wood anatomical features. The Laguncularieae differ in the ratio of vessel member to fibre length, Terminaliinae and Pteleopsidinae cannot be separated wood anatomically. Although difficult to interpret phylogenetically, arguments are brought forward to consider Strephonema ashaving the most primitive wood structure and the Combretinae to have the most derived wood. Variation in some quantitative characters such as vessel member length is shown to be at least partly correlated with ecological conditions of the taxa involved. Wood anatomical differences between lianas and discussed. the of the Combretaceae and erect species are Synoptical keys to genera to the species studied of Terminalia are given. -

Species Richness and Composition During Sylvigenesis in a Tropical Dry Forest in Northwestern Costa Rica
HEINRICH & HURKA 43 Tropical Ecology 45(1): 43-57, 2004 ISSN 0564-3295 © International Society for Tropical Ecology Species richness and composition during sylvigenesis in a tropical dry forest in northwestern Costa Rica ANDREAS HEINRICH & HERBERT HURKA University of Osnabrueck, FB 5 Biology, Spez. Bot. 49069 Osnabrueck, Germany Abstract: Plant species diversity and composition of life form categories were recorded in a tropical dry forest in northwestern Costa Rica. Nine pairs of spatially separated plots were described representing each of different ages of secondary regeneration ranging from recently cleared and burned to more than 50 years of regrowth. All vascular plants species encountered were recorded during 30 months. The results document great changes in species diversity and composition of plant form categories during sylvigenesis. 328 plant species in 79 families and 247 genera of grasses, herbs, shrubs, lianas and trees were encountered. Species richness was greatest after 15 years and decreased significantly in older plots. The number of non-woody species was highest after three years of succession. Minimum of woody plant species was after one year and increased significantly after five years. Maximum was in the 15 years plots with a slight decrease in older forests. Resumen: Se registró la diversidad de especies de plantas y la composición de categorías de formas de vida en un bosque tropical seco del noroeste de Costa Rica. Se describieron nueve pares de parcelas separadas espacialmente que representan diferentes edades en la regeneración secundaria, desde algunas recientemente tumbadas y quemadas hasta otras con crecimiento secundario de más de 50 años. Todas las especies de plantas vasculares que se encontraron fueron registradas durante 30 meses. -

BIODIVERSITY of Pyrrhopyeine SKIPPER BUTTERFLIES (HESPERIIDAE) in the AREA DE CONSERVACION Euanacaste, COSTA RICA Not Once Upon
Journal of the Lepidopterists' Society 55(1), 2001, 15-43 BIODIVERSITY OF PYRRHOPyeINE SKIPPER BUTTERFLIES (HESPERIIDAE) IN THE AREA DE CONSERVACION eUANACASTE, COSTA RICA JOHN M . BURNS Department of Systematic Biology, Entomology Section, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560-0127, USA [email protected] AND DANIEL H. JANZEN Department of Biology, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA [email protected] ABSTRACT. Twenty-two years of rearing 2192 pyrrhopygine caterpillars (and collecting far fewer adults) show that the Area de Conser vacion Guanacaste (ACG) supports at least 15 species, or some 60% of the Costa Rican pyrrhopygine fauna: 3 in (lowest elevation) dry forest Elbella scylla (Menetries) [of which E. dulcinea (Plotz) is a synonym], Mysoria amhigua (Mabille & Boullet), and Myscelus amystis hages God man & Salvin; 4 in (highest elevation) cloud forest- Pyrrhopyge creon H. Druce, P aesculapus Staudinger, P cosyra H. Druce, and Passova gellias (Godman & Salvin); and 10 in (middle elevation) rainforest-Pyrrhopyge zenodorus Godman & Salvin, P crida (Hewitson), P cosyra, P erythrosticta (Godman & Salvin) [lectotype designated], Parelbella macleannani (Godman & Salvin), Jemadia pseudognetus (Mabille) [rein stated status], another Jemadia belonging to aJ. hewitsonii species complex, Myscelus belti Godman & Salvin, M. perissodora Dyar [rein stated status]' and Passova gellias. No species spans all three ecosystems, and none occurs in both dry forest and rainforest. The caterpillars are mostly 0.1-3 m above the ground, even when individuals of their foodplants rise 20-40 m. Except for Myscelus and Passova, the caterpillars are showy because they are ringed, barred, or spotted (with a row of large round dots) in contrasting colors. -

Beyond Hummingbird-Flowers: the Other Side of Ornithophily in the Neotropics
Oecologia Australis 14(1): 67-99, Março 2010 doi:10.4257/oeco.2010.1401.03 BEYOND HUMMINGBIRD-FLOWERS: THE OTHER SIDE OF ORNITHOPHILY IN THE NEOTROPICS Márcia A. Rocca 1* & Marlies Sazima 2 1 Laboratório de Ecologia, Departamento de Ciências Biológicas, Universidade Estadual de Santa Cruz. Campus Soane Nazaré de Andrade, Pavilhão Jorge Amado, Km 16, Rod. Ilhéus-Itabuna. Ilhéus, BA, Brasil. CEP: 45650-000. 2 Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas. Campinas, SP, Brasil. Caixa Postal: 6109. CEP: 13083-970. E-mails: [email protected], [email protected] ABSTRACT Bird pollination in the Neotropical region is by far known as an important interaction to ecosystem function, but perching birds visiting flowers are still very often observed as merely opportunist visitors. Although these other birds do not rely only on floral resources, there are many plant species that do depend solely on them for pollination. These flowers display some features, including morphology and different kinds of resources, quite different from the ornithophilous flowers pollinated by hummingbirds. We review the syndrome of ornithophily in the Neotropical region and tear it apart into the two exploitation methods to determine the floral features that might favor pollination by hovering birds or by perching birds. We listed the Neotropical perching bird species mentioned in the literature as pollinators to take a look at that avian richness. We expect that as more studies on forest canopies are taken in the Neotropics, more interactions between perching birds and flowers may be observed and reveal its real role in the biology of both groups. -

Taxonomy, Phylogeny and Resource Use of Glyptapanteles (Hymenoptera: Braconidae, Microgastrinae), Genus Highly Diversified in the Neotropics
TAXONOMY, PHYLOGENY AND RESOURCE USE OF GLYPTAPANTELES (HYMENOPTERA: BRACONIDAE, MICROGASTRINAE), GENUS HIGHLY DIVERSIFIED IN THE NEOTROPICS BY DIANA CAROLINA ARIAS PENNA DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Entomology in the Graduate College of the University of Illinois at Urbana-Champaign, 2014 Urbana, Illinois Doctoral Committee: Professor James B. Whitfield, Chair, Director of Research Professor May R. Berenbaum Professor Sydney A. Cameron Dr. Marianne Alleyne Dr. Kevin P. Johnson ABSTRACT Among hymenopteran parasitoid wasps, Ichneumonoidea is one of the superfamilies with the highest number of species and includes the highly diverse Microgastrinae subfamily. Microgastrinae wasps are very abundant and can be collected in many different terrestrial habitats. A considerable number of species have been used in successful biological pest control programs, making it one of the most important insect groups. Glyptapanteles is one of the larger genera within Microgastrinae, which was segregated after several attempts to subdivide the gigantic genus Apanteles Foerster 1862. To date, 122 species have been described worldwide, of which only six are Neotropical, despite unpublished evidence that this genus is one of the largest in the Neotropics. Glyptapanteles are diminutive parasitoid wasps, which are free-living as adults, but as immatures, they attack exclusively larvae of Lepidoptera as a food resource for their developing larvae. These parasitoid wasps play a preponderant role in regulating their lepidopteran host populations and in maintaining high biological diversity in terrestrial ecosystems. A reliable revision for the Neotropics has not yet been attempted and many Glyptapanteles species remain undescribed. The scarcity of both taxon sampling and biological information is no longer standard for some Neotropical groups of insects. -

Combretaceae
GUIDE TO THE GENERA OF LIANAS AND CLIMBING PLANTS IN THE NEOTROPICS COMBRETACEAE By Pedro Acevedo-Rodríguez (Apr 2020) A family of trees, shrubs and lianas with pantropical distribution, some members extending to warm temperate zones. Worldwide, the family consists of 11 genera and about 400 species, of which only Combretum, Getonia and Meiostemon contain lianas. In the Neotropics, the family is represented by 4 genera and about 85 species, of which only Combretum has 33 species of climbing plants. The genus is generally found at between 200-800 m elevation and occurs in wet or gallery forests. Diagnostics: Species of Combretum are twining lianas with simple, opposite, exstipulate leaves, and often prominent axillary buds; flowers often showy, Combretum grandiflorum, photo by P. Acevedo 4-5-merous, with tubular, infundibuliform or bell- shaped hypanthium, and very long and showy stamens; fruits 4- or 5- winged. Many species produce a mucilaginous exudate when the stem is cut. In the absence of flowers, Combretaceae lianas may be confused with Malpighiaceae lianas as they both have twining stems and simple opposite leaves. However, Combretaceae is easily distinguished by the absence of stipules or petiolar glands, by the presence of intraxylary phloem, and the presence of sclerenchyma bands in the cortex (vs. stipulate, often with petiolar and laminar glands, absence of intraxylary phloem, and sclerenchyma bundles in the cortex). General Characters 1. STEMS. Branches are cylindrical or quadrangular; mature stems are cylindrical, in some species known to reach up to 9 cm in diam. and 20 m in length, sometimes provided with alternate straight spines. -
Mixtec Plant Nomenclature and Classification by Alejandro De Ávila a Dissertation Submitted in Partial Satisfaction of The
Mixtec plant nomenclature and classification by Alejandro de Ávila A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor in Philosophy in Anthropology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Overton Brent Berlin, Chair Professor Laura Nader Professor Leanne Hinton Fall 2010 Abstract Mixtec plant nomenclature and classification by Alejandro de Ávila Doctor of Philosophy in Anthropology University of California, Berkeley Professor Overton Brent Berlin, Chair Ñuu Savi (‘Sacred Rain’s collectivity’), the Mixtec people of southern Mexico, had created some of the most complex polities in the continent at the time of European contact. Five hundred years later, they remain cohesive, culturally distinct communities, as increasing numbers of individuals and families migrate to northern Mexico and the US for work in the agricultural and service sectors. In 2005, the Mexican Federal Government reported there were more than 446,000 speakers of Tu’un Savi (‘Sacred Rain’s word,’ the Mixtec languages) five years of age and older, 322,000 of them still living in 1551 settlements within their historic homeland; an additional 100,000 to 200,000 are estimated to reside in the US. The term Mixtec, derived from the Náhuatl mixte:cah (‘cloud-people’), has been considered by different authors to encompass between 12 and 52 mutually unintelligible languages, in addition to numerous dialects. According to the Summer Institute of Linguistics’ Ethnologue, it is the second most diversified group of languages in the Americas, after Zapotec. The Instituto Nacional de Lenguas Indígenas, however, recognizes 81 variants of Mixtec, making it the most diversified language group in Mexico following official criteria. -
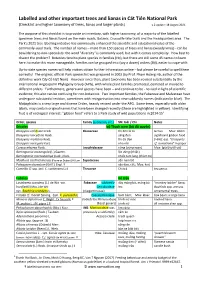
Labelled and Other Important Trees and Lianas in Cát Tiên National Park (Checklist and Higher Taxonomy of Trees, Lianas and Larger Plants) V.2 Update: 18 August 2021
Labelled and other important trees and lianas in Cát Tiên National Park (Checklist and higher taxonomy of trees, lianas and larger plants) v.2 update: 18 August 2021 The purpose of this checklist is to provide an inventory, with higher taxonomy, of a majority of the labelled specimen trees and lianas found on the main roads, Botanic, Crocodile-lake trails and the Headquarters area. The Park's 2021 tree labelling initiative has enormously enhanced the scientific and educational value of the commonly-used trails. The number of names – more than 150 species of trees and lianas (woody vines) - can be bewildering to non-specialists: the word "diversity" is commonly used, but with it comes complexity. How best to dissect the problem? Botanists tend to place species in families (Họ), but there are still some 45 names to learn here; to make this more manageable, families can be grouped into (say a dozen) orders (Bộ), easier to cope-with. Up-to-date species names will help visitors obtain further information online – but please be careful to spell them correctly! The original, official Park species list was prepared in 2002 (by Prof. Phạm Hoàng Hộ, author of the definitive work Cây Cỏ Việt Nam). However since then, plant taxonomy has been revised substantially by the international Angiosperm Phylogeny Group (APG), with whole plant families promoted, demoted or moved to different orders. Furthermore, genera and species have been – and continue to be - revised in light of scientific evidence; this also can be confusing for non-botanists. Two important families, the Fabaceae and Malvaceae have undergone substantial revision, sometimes with reorganisation into new subfamily names (indicated in blue).