How We Manage Bone Marrow Edema—An Interdisciplinary Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Investigating the Genetic and Genomic Basis of Osteochondrosis in Thoroughbred Horses from Australia and New Zealand
Investigating the genetic and genomic basis of osteochondrosis in Thoroughbred horses from Australia and New Zealand Kao Castle The University of Sydney A thesis submitted to the Faculty of Veterinary Science, The University of Sydney, in fulfilment of the requirements for the Degree of Doctor of Philosophy December 2012 i Declaration I declare that the work presented in this thesis is, to the best of my knowledge and belief, original, except as acknowledged in the text, and that the material has not been submitted, either in whole or in part, for a degree at this or any other university. (Kao Castle) 17 December 2012 ii Statement of Contributions The identities of the studs and horses that participated in the research described in this thesis are protected by confidentiality agreements. On this basis, the names of stud staff and veterinarians who provided data for this research are not listed as authors or named in the acknowledgements, although their input was invaluable. Chapter 2, Skeletal lesions and injuries in Australasian Thoroughbred weanling and yearling radiographs (draft manuscript). Castle K., Tammen I., Thomson P.C., Jeffcott L.B., Raadsma H.W. and Nicholas F.W. • Dr Tammen supervised components of this work, contributed to discussions, and provided feedback on the presentation of results. • Associate Professor Thomson provided advice on statistical techniques to compare results between the weanling and yearling populations, and provided feedback on the presentation of results. • Professors Jeffcott and Raadsma contributed to planning the data collection strategy, and provided feedback on the presentation of results. • Emeritus Professor Nicholas supervised components of this work, contributed to discussions and provided extensive feedback on the presentation of the results, and edited the manuscript. -

ICRS Heritage Summit 1
ICRS Heritage Summit 1 20th Anniversary www.cartilage.org of the ICRS ICRS Heritage Summit June 29 – July 01, 2017 Gothia Towers, Gothenburg, Sweden Final Programme & Abstract Book #ICRSSUMMIT www.cartilage.org Picture Copyright: Zürich Tourismus 2 The one-step procedure for the treatment of chondral and osteochondral lesions Aesculap Biologics Facing a New Frontier in Cartilage Repair Visit Anika at Booth #16 Easy and fast to be applied via arthroscopy. Fixation is not required in most cases. The only entirely hyaluronic acid-based scaffold supporting hyaline-like cartilage regeneration Biologic approaches to tissue repair and regeneration represent the future in healthcare worldwide. Available Sizes Aesculap Biologics is leading the way. 2x2 cm Learn more at www.aesculapbiologics.com 5x5 cm NEW SIZE Aesculap Biologics, LLC | 866-229-3002 | www.aesculapusa.com Aesculap Biologics, LLC - a B. Braun company Website: http://hyalofast.anikatherapeutics.com E-mail: [email protected] Telephone: +39 (0)49 295 8324 ICRS Heritage Summit 3 The one-step procedure for the treatment of chondral and osteochondral lesions Visit Anika at Booth #16 Easy and fast to be applied via arthroscopy. Fixation is not required in most cases. The only entirely hyaluronic acid-based scaffold supporting hyaline-like cartilage regeneration Available Sizes 2x2 cm 5x5 cm NEW SIZE Website: http://hyalofast.anikatherapeutics.com E-mail: [email protected] Telephone: +39 (0)49 295 8324 4 Level 1 Study Proves Efficacy of ACP in -

Hypophosphatasia Could Explain Some Atypical Femur Fractures
Hypophosphatasia Could Explain Some Atypical Femur Fractures What we know Hypophosphatasia (HPP) is a rare genetic disease that affects the development of bones and teeth in children (Whyte 1985). HPP is caused by the absence or reduced amount of an enzyme called tissue-nonspecific alkaline phosphatase (TAP), also called bone-specific alkaline phosphatase (BSAP). The absence of TAP raises the level of inorganic pyrophosphate (Pi), which prevents calcium and phosphate from creating strong, mineralized bone. Without TAP, bones can become weak. In its severe form, HPP is fatal and happens in 1/100,000 births. Because HPP is genetic, it can appear in adults as well. A recent study has identified a milder, more common form of HPP that occurs in 4 of 1000 adults (Dahir 2018). This form of HPP is usually seen in early middle aged adults who have low bone density and sometimes have stress fractures in the feet or thigh bone. Sometimes these patients lose their baby teeth early, but not always. HPP is diagnosed by measuring blood levels of TAP and vitamin B6. An elevated vitamin B6 level [serum pyridoxal 5-phosphate (PLP)] (Whyte 1985) in a patient with a TAP level ≤40 or in the low end of normal can be diagnosed with HPP. Almost half of the adult patients with HPP in the large study had TAP >40, but in the lower end of the normal range (Dahir 2018). The connection between hypophosphatasia and osteoporosis Some people who have stress fractures get a bone density test and are treated with an osteoporosis medicine if their bone density results are low. -

Occasional Articles Histology of Normal Haemopoiesis: Bone Marrow
I Clin Pathol 1992;45:645-649 645 Occasional articles Histology of normal haemopoiesis: Bone marrow J Clin Pathol: first published as 10.1136/jcp.45.8.645 on 1 August 1992. Downloaded from histology I B S Wilkins Introduction trabecular spaces,2 but little is known about The bone marrow trephine biopsy specimen the dynamics of blood flow through human holds an unusual position among pathological bone marrow. Arterioles and venules tend to lie specimens. Having been obtained in most towards the centres of intertrabecular spaces. cases by a haematologist, the biopsy specimen They are usually seen in only a small propor- is processed in a histopathology laboratory and tion of intertrabecular spaces in any one biopsy is then reported either by a haematologist or a specimen, suggesting that each may supply or histopathologist, depending on local custom. drain a number of such spaces. Individual practitioners may acquire great skill The trabeculae, arterioles, and venules form in the interpretation of trephine biopsy speci- the structural framework around which mens, but others in both branches ofpathology granulopoiesis develops. Erythropoiesis and are sometimes perplexed when faced with megakaryopoiesis occur in apposition to the tissue appearances for which other experience fine, branching sinusoids. within their individual disciplines may not have prepared them fully. Ideally, interpretation MARROW STROMA should be a collaborative procedure, combin- The trabecular and vascular architecture of ing the clinical and cytological knowledge of medullary bone provides the basic framework, the haematologist with the histopathologist's nutritional supply, and waste removal system skills in analysis ofnormal and abnormal tissue for haemopoiesis, but specialised support of structure. -

Royal Entomological Society
Royal Entomological Society HANDBOOKS FOR THE IDENTIFICATION OF BRITISH INSECTS To purchase current handbooks and to download out-of-print parts visit: http://www.royensoc.co.uk/publications/index.htm This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 2.0 UK: England & Wales License. Copyright © Royal Entomological Society 2012 ROYAL ENTOMOLOGICAL , SOCIETY OF LONDON Vol. I. Part 1 (). HANDBOOKS FOR THE IDENTIFICATION OF BRITISH INSECTS SIPHONAPTERA 13y F. G. A. M. SMIT LONDON Published by the Society and Sold at its Rooms - 41, Queen's Gate, S.W. 7 21st June, I9S7 Price £1 os. od. ACCESSION NUMBER ....... ................... British Entomological & Natural History Society c/o Dinton Pastures Country Park, Davis Street, Hurst, OTS - Reading, Berkshire RG 10 OTH .•' Presented by Date Librarian R EGULATIONS I.- No member shall be allowed to borrow more than five volumes at a time, or to keep any of tbem longer than three months. 2.-A member shall at any time on demand by the Librarian forthwith return any volumes in his possession. 3.-Members damaging, losing, or destroying any book belonging to the Society shall either provide a new copy or pay such sum as tbe Council shall tbink fit. ) "1' > ) I .. ··•• · ·• "V>--· .•. .t ... -;; ·· · ·- ~~- -~· · · ····· · · { · · · l!i JYt.11'ian, ,( i-es; and - REGU--LATIONS dthougll 1.- Books may b - ~dapted, ; ~ 2 -~ . e borrowed at . !.l :: - --- " . ~ o Member shall b . all Meeflfll(s of the So J t Volumes at a time o; ,IJJowed to borrow more c e y . 3.- An y Mem ber r t '. to keep them lonl{er th than three b.ecorn_e SPecified f e a Jn!ng a \'oJume a n one m on th. -
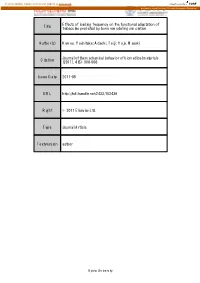
Effects of Loading Frequency on the Functional Adaptation of Title Trabeculae Predicted by Bone Remodeling Simulation
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository Effects of loading frequency on the functional adaptation of Title trabeculae predicted by bone remodeling simulation. Author(s) Kameo, Yoshitaka; Adachi, Taiji; Hojo, Masaki Journal of the mechanical behavior of biomedical materials Citation (2011), 4(6): 900-908 Issue Date 2011-08 URL http://hdl.handle.net/2433/152436 Right © 2011 Elsevier Ltd. Type Journal Article Textversion author Kyoto University Effects of loading frequency on the functional adaptation of trabeculae predicted by bone remodeling simulation Yoshitaka Kameoa, b, Taiji Adachib, c, and Masaki Hojoa a: Department of Mechanical Engineering and Science, Kyoto University b: Computational Cell Biomechanics Team, VCAD System Research Program, RIKEN c: Department of Biomechanics, Institute for Frontier Medical Sciences, Kyoto University Corresponding author: Taiji Adachi, Ph.D. Mailing Address: Department of Biomechanics, Institute for Frontier Medical Sciences, Kyoto University 53, Syogoin-kawaramachi, Sakyo, Kyoto 606-8507, Japan Telephone & Fax: +81 (75) 751-4853 E-mail: [email protected] Submitted to Journal of the Mechanical Behavior of Biomedical Materials Key words: Bone remodeling, Functional adaptation, Loading frequency, Cellular mechanosensing, Computational simulation 1 Abstract The process of bone remodeling is regulated by metabolic activities of many bone cells. While osteoclasts and osteoblasts are responsible for bone resorption and formation, respectively, activities of these cells are believed to be controlled by a mechanosensory system of osteocytes embedded in the extracellular bone matrix. Several experimental and theoretical studies have suggested that the strain-derived interstitial fluid flow in lacuno-canalicular porosity serves as the prime mover for bone remodeling. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Establishment of a Dental Effects of Hypophosphatasia Registry Thesis
Establishment of a Dental Effects of Hypophosphatasia Registry Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Jennifer Laura Winslow, DMD Graduate Program in Dentistry The Ohio State University 2018 Thesis Committee Ann Griffen, DDS, MS, Advisor Sasigarn Bowden, MD Brian Foster, PhD Copyrighted by Jennifer Laura Winslow, D.M.D. 2018 Abstract Purpose: Hypophosphatasia (HPP) is a metabolic disease that affects development of mineralized tissues including the dentition. Early loss of primary teeth is a nearly universal finding, and although problems in the permanent dentition have been reported, findings have not been described in detail. In addition, enzyme replacement therapy is now available, but very little is known about its effects on the dentition. HPP is rare and few dental providers see many cases, so a registry is needed to collect an adequate sample to represent the range of manifestations and the dental effects of enzyme replacement therapy. Devising a way to recruit patients nationally while still meeting the IRB requirements for human subjects research presented multiple challenges. Methods: A way to recruit patients nationally while still meeting the local IRB requirements for human subjects research was devised in collaboration with our Office of Human Research. The solution included pathways for obtaining consent and transferring protected information, and required that the clinician providing the clinical data refer the patient to the study and interact with study personnel only after the patient has given permission. Data forms and a custom database application were developed. Results: The registry is established and has been successfully piloted with 2 participants, and we are now initiating wider recruitment. -

Metabolic Bone Disease 5
g Metabolic Bone Disease 5 Introduction, 272 History and examination, 275 Osteoporosis, 283 STRUCTURE AND FUNCTION, 272 Investigation, 276 Paget’s disease of bone, 288 Structure of bone, 272 Management, 279 Hyperparathyroidism, 290 Function of bone, 272 DISEASES AND THEIR MANAGEMENT, 280 Hypercalcaemia of malignancy, 293 APPROACH TO THE PATIENT, 275 Rickets and osteomalacia, 280 Hypocalcaemia, 295 Introduction Calcium- and phosphate-containing crystals: set in a structure• similar to hydroxyapatite and deposited in holes Metabolic bone diseases are a heterogeneous group of between adjacent collagen fibrils, which provide rigidity. disorders characterized by abnormalities in calcium At least 11 non-collagenous matrix proteins (e.g. osteo- metabolism and/or bone cell physiology. They lead to an calcin,• osteonectin): these form the ground substance altered serum calcium concentration and/or skeletal fail- and include glycoproteins and proteoglycans. Their exact ure. The most common type of metabolic bone disease in function is not yet defined, but they are thought to be developed countries is osteoporosis. Because osteoporosis involved in calcification. is essentially a disease of the elderly, the prevalence of this condition is increasing as the average age of people Cellular constituents in developed countries rises. Osteoporotic fractures may lead to loss of independence in the elderly and is imposing Mesenchymal-derived osteoblast lineage: consist of an ever-increasing social and economic burden on society. osteoblasts,• osteocytes and bone-lining cells. Osteoblasts Other pathological processes that affect the skeleton, some synthesize organic matrix in the production of new bone. of which are also relatively common, are summarized in Osteoclasts: derived from haemopoietic precursors, Table 3.20 (see Chapter 4). -

Osteochondrosis and Arthrosis in Pigs Ii
Acta vet. scand, 1974, 15, 26-42. From the Department of Pathology, Veterinary College of Norway, Oslo. OSTEOCHONDROSIS AND ARTHROSIS IN PIGS II. INCIDENCE IN BREEDING ANIMALS By Trygve Grendalen GR0NDALEN, TRYGVE: Osteochondrosis and arthrosis in pigs. 11. Incidence in breeding animals. Acta vet. scand. 1974, 15, 26-42. - Joint and bone lesions in breeding pigs are described on the background of an investdgatlon involving 174 sows and 155 boars from 7 months to 4% years old . Lesions, which consisted pre dominantly of arthrosis, degeneration of intervertebral discs, spon dylosis and epiphyseal separations, were demonstrated frequently in both sexes. Osteochondrosis, a condition previously demonstrated frequently in slaughter pigs, had either completely healed, undergone repair or developed into an arthrosis by the time the animal reached an age of about 1% years. Whereas a higher incidence of arthrosis of the intervertebral joints was found in boars than in sows, the reverse was true as regards degeneration of the intervertebral discs and anchylosing spondylosis. Possible reasons for this are discussed. Norwegian pigs show a higher incidence of lesions in the lumbar region of the vertebral column than has been described up to the present time in other countries. os teo c h 0 n d r 0 sis; art h r 0 sis; pig. A high incidence of osteochondrosis in joints and epiphyseal plates, and arthrosis, especially in the lumbar intervertebral joints, the distomedial hock joints, the elbow and stifle joint in pigs up to 120 kg live weight has been previously described (Grpndalen 1974). A review of the literature showed that osteo chondrosisand archrosis are widespread in pigs in various coun tries. -

Osteochondrosis – Primary Epiphyseal (Articular/Subchondral) Lesion Can Heal Or Can Progress
60 120 180 1 distal humeral condyles 2 medial epicondyle 3 proximal radial epiphysis 4 anconeal process Lab Ret study N=1018 . Normal . Affected . Total 688 (67.6%) . Total 330 (32.4%) . Male 230 (62.2%) . Male 140 (37.8%) . Female 458 (70.7%) . Female 190 (29.3%) Affected dogs N=330 1affected site - 250 (75.7%) 2 affected sites - 68 (20.6%) 3 affected sites - 12 (3.6%) immature skeletal diseases denis novak technique for skeletal radiography tissue < 12 cm “non-grid” (“table-top”) technique “high detail” system radiation safety diagnosis X – rays examination Ultrasound CT bilateral lesions - clinical signs ? unilateral present > one type of lesion 2ry arthrosis Common Osteochondrosis – primary epiphyseal (articular/subchondral) lesion can heal or can progress Osteochondritis dissecans – free articular fragment will progress Arthrosis Osteochondrosis talus / tarsus Lumbosacral OCD Lumbosacral OCD Inflammatory diseases Panosteitis – non infectious Hypertrophic osteodystrophy (HOD) – perhaps infectious Osteomyelitis - infectious Panosteitis New medullary bone Polyostotic Multiple lesions in one bone Symmetrical or nonsymmetrical Sclerotic pattern B I L A T E R A L periosteal new bone forms with chronicity Cross sections of a tibia different locations Hypertrophic osteodystrophy (HOD) Dogs are systemically ill, febrile, anorectic, reluctant to walk most will recover Radiographic changes of HOD . Polyostotic . Metaphyseal . Symmetrical . Changes of lesion Early Mid Late lytic “plates” in acute case HOD - 4 m ret – lesions are present