Download.Html) and a Pairwise Identity Percentage 145 of 0.97
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heat Resistant Thermophilic Endospores in Cold Estuarine Sediments
Heat resistant thermophilic endospores in cold estuarine sediments Emma Bell Thesis submitted for the degree of Doctor of Philosophy School of Civil Engineering and Geosciences Faculty of Science, Agriculture and Engineering February 2016 Abstract Microbial biogeography explores the spatial and temporal distribution of microorganisms at multiple scales and is influenced by environmental selection and passive dispersal. Understanding the relative contribution of these factors can be challenging as their effects can be difficult to differentiate. Dormant thermophilic endospores in cold sediments offer a natural model for studies focusing on passive dispersal. Understanding distributions of these endospores is not confounded by the influence of environmental selection; rather their occurrence is due exclusively to passive transport. Sediment heating experiments were designed to investigate the dispersal histories of various thermophilic spore-forming Firmicutes in the River Tyne, a tidal estuary in North East England linking inland tributaries with the North Sea. Microcosm incubations at 50-80°C were monitored for sulfate reduction and enriched bacterial populations were characterised using denaturing gradient gel electrophoresis, functional gene clone libraries and high-throughput sequencing. The distribution of thermophilic endospores among different locations along the estuary was spatially variable, indicating that dispersal vectors originating in both warm terrestrial and marine habitats contribute to microbial diversity in estuarine and marine environments. In addition to their persistence in cold sediments, some endospores displayed a remarkable heat-resistance surviving multiple rounds of autoclaving. These extremely heat-resistant endospores are genetically similar to those detected in deep subsurface environments, including geothermal groundwater investigated from a nearby terrestrial borehole drilled to >1800 m depth with bottom temperatures in excess of 70°C. -

WO 2018/064165 A2 (.Pdf)
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/064165 A2 05 April 2018 (05.04.2018) W !P O PCT (51) International Patent Classification: Published: A61K 35/74 (20 15.0 1) C12N 1/21 (2006 .01) — without international search report and to be republished (21) International Application Number: upon receipt of that report (Rule 48.2(g)) PCT/US2017/053717 — with sequence listing part of description (Rule 5.2(a)) (22) International Filing Date: 27 September 2017 (27.09.2017) (25) Filing Language: English (26) Publication Langi English (30) Priority Data: 62/400,372 27 September 2016 (27.09.2016) US 62/508,885 19 May 2017 (19.05.2017) US 62/557,566 12 September 2017 (12.09.2017) US (71) Applicant: BOARD OF REGENTS, THE UNIVERSI¬ TY OF TEXAS SYSTEM [US/US]; 210 West 7th St., Austin, TX 78701 (US). (72) Inventors: WARGO, Jennifer; 1814 Bissonnet St., Hous ton, TX 77005 (US). GOPALAKRISHNAN, Vanch- eswaran; 7900 Cambridge, Apt. 10-lb, Houston, TX 77054 (US). (74) Agent: BYRD, Marshall, P.; Parker Highlander PLLC, 1120 S. Capital Of Texas Highway, Bldg. One, Suite 200, Austin, TX 78746 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Metal Transformation by a Novel Pelosinus Isolate from a Subsurface Environment
INL/JOU-08-14091-Revision-0 Metal Transformation by a Novel Pelosinus Isolate From a Subsurface Environment Allison E. Ray, Peter P. Sheridan, Andrew L. Neal, Yoshiko Fujita, David E. Cummings, Timothy S. Magnuson August 2018 The INL is a U.S. Department of Energy National Laboratory operated by Battelle Energy Alliance INL/JOU-08-14091-Revision-0 Metal Transformation by a Novel Pelosinus Isolate From a Subsurface Environment Allison E. Ray, Peter P. Sheridan, Andrew L. Neal, Yoshiko Fujita, David E. Cummings, Timothy S. Magnuson August 2018 Idaho National Laboratory Idaho Falls, Idaho 83415 http://www.inl.gov Prepared for the U.S. Department of Energy Office of Nuclear Energy Under DOE Idaho Operations Office Contract DE-AC07-05ID14517 fmicb-09-01689 July 25, 2018 Time: 20:42 # 1 ORIGINAL RESEARCH published: xx July 2018 doi: 10.3389/fmicb.2018.01689 1 58 2 59 3 60 4 61 5 62 6 63 7 64 8 65 9 Metal Transformation by a Novel 66 10 67 11 Pelosinus Isolate From a Subsurface 68 12 69 13 Environment 70 14 71 15 Allison E. Ray1,2, Stephanie A. Connon1,3, Andrew L. Neal4†, Yoshiko Fujita2, 72 5 2† 1 16 David E. Cummings , Jani C. Ingram and Timothy S. Magnuson * 73 17 74 1 Department of Biological Sciences, Idaho State University, Pocatello, ID, United States, 2 Bioenergy Technologies, Idaho 18 3 75 Edited by: National Laboratory, Idaho Falls, ID, United States, California Institute of Technology, Pasadena, CA, United States, 19 4 5 76 Pankaj Kumar Arora, Savannah River Ecology Laboratory, University of Georgia, Aiken, SC, United States, Department of Biology, Point Loma 20 77 Babasaheb Bhimrao Ambedkar Nazarene University, San Diego, CA, United States 21 University, India 78 22 79 Reviewed by: The capability of microorganisms to alter metal speciation offers potential for 23 80 Ramprasad E.V.V., the development of new strategies for immobilization of toxic metals in the 24 University of Hyderabad, India 81 25 Bärbel Ulrike Fösel, environment. -
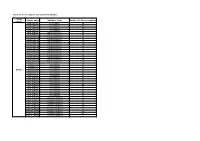
Table S1: List of Samples Included in the Analysis
Table S1: list of samples included in the analysis Study Sample name Inhibitory status Number of days at sampling number DNA.0P2T4 No inhibition 29 DNA.0P2T6 No inhibition 57 DNA.10P2T4 No inhibition 29 DNA.10P2T6 No inhibition 57 DNA.75P2T4 Phenol inhibition 29 DNA.75P2T6 Phenol inhibition 57 DNA.100P2T4 Phenol inhibition 29 DNA.100P2T6 Phenol inhibition 57 DNA.125P1T4 Phenol inhibition 29 DNA.125P1T6 Phenol inhibition 57 DNA.125P2T4 Phenol inhibition 29 DNA.125P2T6 Phenol inhibition 57 DNA.125P3T4 Phenol inhibition 29 DNA.125P3T6 Phenol inhibition 57 DNA.150P2T4 Phenol inhibition 29 DNA.150P2T6 Phenol inhibition 57 DNA.200P2T4 Phenol inhibition 29 DNA.200P2T6 Phenol inhibition 57 DNA.0N2T4 No inhibition 29 DNA.0N2T5 No inhibition 42 DNA.0N2T6 No inhibition 57 Study 1 DNA.5N2T4 No inhibition 29 DNA.5N2T5 No inhibition 42 DNA.5N2T6 No inhibition 57 DNA.10N2T4 No inhibition 29 DNA.10N2T5 No inhibition 42 DNA.10N2T6 No inhibition 57 DNA.15N2T4 No inhibition 29 DNA.15N2T5 No inhibition 42 DNA.15N2T6 No inhibition 57 DNA.25N2T4 No inhibition 29 DNA.25N2T5 No inhibition 42 DNA.25N2T6 No inhibition 57 DNA.75N2T4 Ammonia inhibition 29 DNA.75N2T5 Ammonia inhibition 42 DNA.75N2T6 Ammonia inhibition 57 DNA.100N2T4 Ammonia inhibition 29 DNA.100N2T5 Ammonia inhibition 42 DNA.100N2T6 Ammonia inhibition 57 DNA.250N2T4 Ammonia inhibition 29 DNA.250N2T5 Ammonia inhibition 42 DNA.250N2T6 Ammonia inhibition 57 nono2T3 No inhibition 16 noN2T4 Ammonia inhibition 23 noN2T8 Ammonia inhibition 60 noN2T9 Ammonia inhibition 85 noPhi2T4 Phenol inhibition 23 noPhi2T5 -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Corrinoid Cross-Feeding in the Microbial World Permalink https://escholarship.org/uc/item/2z66b95b Author Dirks, Erica Christine Publication Date 2014 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Corrinoid Cross-Feeding in the Microbial World By Erica Christine Dirks A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Microbiology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Michiko E. Taga, Chair Professor John D. Coates Professor David Savage Professor Nicole King Fall 2014 Acknowledgements This work would not have been possible without the generous and kind support of many people over the past seven years. To all of my collaborators and mentors I’ve met along the way, especially the members of the Taga lab, a million thanks for all of the advice, discussions, and guidance. Your unselfish willingness to help continues to amaze and inspire me. Working with you has been a true gift. To Shan, I’ve learned so much from you. I wish you every happiness, and I will always think of you as my big sister in science. To Steve, it’s meant so much to me to have you on my side. Your breadth of knowledge astounds me. I hope you never stop telling stories. To Patrick, you are a true friend, and I would have never gotten through without you. To Jerome, together, you know we can defeat the zombie hordes. Thanks for all the car rides! To Mark, for pushing me to go further than I ever imagined I could. -

Physiologica Thermoa Al and Phylogenetic Stu Caloramator And
University of Akureyri Faculty of Business and Science Department of Natural Resource Sciences Physiological and phylogenetic studies of Caloramator and Thermoanaerobacterium species: Ethanol and hydrogen production from complex biomass Margrét Auður Sigurbjörnsdóttir Supervisor: Jóhann Örlygsson University of Akureyri Submitted in part fulfillment of the degree of Master of Science in Natural Resource Science – Biotechnology May 2009 University of Akureyri Department of Business and Science “There are in fact two things, science and opinion; the former begets knowledge, the latter ignorance.” Hippocrates ii University of Akureyri Department of Business and Science University of Akureyri Faculty of Business and Science Department of Natural Resource Sciences Physiological and phylogenetic studies of Caloramator and Thermoanaerobacterium species: Ethanol and hydrogen production from complex biomass Margrét Auður Sigurbjörnsdóttir Master thesis for 90 credit M.Sc. in Biotechnology Printed copies: 10 Number of pages: 109 Appendixes: 2 iii University of Akureyri Department of Business and Science Declaration I hereby declare that I am the only author of this thesis and it is the product of my own research. _______________________________________________ Margrét Auður Sigurbjörnsdóttir It is hereby confirmed that this master thesis is satisfactory to M.Sc. – degree from the Faculty of Business and Science, department of Natural Resource Science. ________________________________________________ Dr. Jóhann Örlygsson iv University of Akureyri Department of Business and Science Abstract Three anerobic, thermophilic bacteria were isolated from hot springs in Graendalur, SW-Iceland. The strains were investigated with respect to phylogenetic, physiology and end product formation. Phylogenetic studies were done with both partial and full 16S rRNA sequence analysis on all three strains. Two of the strains, AK44 and AK35, belong to the genus Caloramator with the closest relation to C. -

EXPERIMENTAL STUDIES on FERMENTATIVE FIRMICUTES from ANOXIC ENVIRONMENTS: ISOLATION, EVOLUTION, and THEIR GEOCHEMICAL IMPACTS By
EXPERIMENTAL STUDIES ON FERMENTATIVE FIRMICUTES FROM ANOXIC ENVIRONMENTS: ISOLATION, EVOLUTION, AND THEIR GEOCHEMICAL IMPACTS By JESSICA KEE EUN CHOI A dissertation submitted to the School of Graduate Studies Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Microbial Biology Written under the direction of Nathan Yee And approved by _______________________________________________________ _______________________________________________________ _______________________________________________________ _______________________________________________________ New Brunswick, New Jersey October 2017 ABSTRACT OF THE DISSERTATION Experimental studies on fermentative Firmicutes from anoxic environments: isolation, evolution and their geochemical impacts by JESSICA KEE EUN CHOI Dissertation director: Nathan Yee Fermentative microorganisms from the bacterial phylum Firmicutes are quite ubiquitous in subsurface environments and play an important biogeochemical role. For instance, fermenters have the ability to take complex molecules and break them into simpler compounds that serve as growth substrates for other organisms. The research presented here focuses on two groups of fermentative Firmicutes, one from the genus Clostridium and the other from the class Negativicutes. Clostridium species are well-known fermenters. Laboratory studies done so far have also displayed the capability to reduce Fe(III), yet the mechanism of this activity has not been investigated -

Supplementary Figure Legends for Rands Et Al. 2019
Supplementary Figure legends for Rands et al. 2019 Figure S1: Display of all 485 prophage genome maps predicted from Gram-Negative Firmicutes. Each horizontal line corresponds to an individual prophage shown to scale and color-coded for annotated phage genes according to the key displayed in the right- side Box. The left vertical Bar indicates the Bacterial host in a colour code. Figure S2: Projection of virome sequences from 183 human stool samples on A. Acidaminococcus intestini RYC-MR95, and B. Veillonella parvula UTDB1-3. The first panel shows the read coverage (Y-axis) across the complete Bacterial genome sequence (X-axis; with bp coordinates). Predicted prophage regions are marked with red triangles and magnified in the suBsequent panels. Virome reads projected outside of prophage prediction are listed in Table S4. Figure S3: The same display of virome sequences projected onto Bacterial genomes as in Figure S2, But for two different Negativicute species: A. Dialister Marseille, and B. Negativicoccus massiliensis. For non-phage peak annotations, see Table S4. Figure S4: Gene flanking analysis for the lysis module from all prophages predicted in all the different Bacterial clades (Table S2), a total of 3,462 prophages. The lysis module is generally located next to the tail module in Firmicute prophages, But adjacent to the packaging (terminase) module in Escherichia phages. 1 Figure S5: Candidate Mu-like prophage in the Negativicute Propionispora vibrioides. Phage-related genes (arrows indicate transcription direction) are coloured and show characteristics of Mu-like genome organization. Figure S6: The genome maps of Negativicute prophages harbouring candidate antiBiotic resistance genes MBL (top three Veillonella prophages) and tet(32) (bottom Selenomonas prophage remnant); excludes the ACI-1 prophage harbouring example characterised previously (Rands et al., 2018). -

Genome Diversity of Spore-Forming Firmicutes MICHAEL Y
Genome Diversity of Spore-Forming Firmicutes MICHAEL Y. GALPERIN National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health, Bethesda, MD 20894 ABSTRACT Formation of heat-resistant endospores is a specific Vibrio subtilis (and also Vibrio bacillus), Ferdinand Cohn property of the members of the phylum Firmicutes (low-G+C assigned it to the genus Bacillus and family Bacillaceae, Gram-positive bacteria). It is found in representatives of four specifically noting the existence of heat-sensitive vegeta- different classes of Firmicutes, Bacilli, Clostridia, Erysipelotrichia, tive cells and heat-resistant endospores (see reference 1). and Negativicutes, which all encode similar sets of core sporulation fi proteins. Each of these classes also includes non-spore-forming Soon after that, Robert Koch identi ed Bacillus anthracis organisms that sometimes belong to the same genus or even as the causative agent of anthrax in cattle and the species as their spore-forming relatives. This chapter reviews the endospores as a means of the propagation of this orga- diversity of the members of phylum Firmicutes, its current taxon- nism among its hosts. In subsequent studies, the ability to omy, and the status of genome-sequencing projects for various form endospores, the specific purple staining by crystal subgroups within the phylum. It also discusses the evolution of the violet-iodine (Gram-positive staining, reflecting the pres- Firmicutes from their apparently spore-forming common ancestor ence of a thick peptidoglycan layer and the absence of and the independent loss of sporulation genes in several different lineages (staphylococci, streptococci, listeria, lactobacilli, an outer membrane), and the relatively low (typically ruminococci) in the course of their adaptation to the saprophytic less than 50%) molar fraction of guanine and cytosine lifestyle in a nutrient-rich environment. -

Harnessing Synthetic Biology for the Bioprospecting and Engineering of Aromatic Polyketide Synthases
HARNESSING SYNTHETIC BIOLOGY FOR THE BIOPROSPECTING AND ENGINEERING OF AROMATIC POLYKETIDE SYNTHASES A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy in the Faculty of Science and Engineering (2017) MATTHEW J. CUMMINGS SCHOOL OF CHEMISTRY 1 THIS IS A BLANK PAGE 2 List of contents List of contents .............................................................................................................................. 3 List of figures ................................................................................................................................. 8 List of supplementary figures ...................................................................................................... 10 List of tables ................................................................................................................................ 11 List of supplementary tables ....................................................................................................... 11 List of boxes ................................................................................................................................ 11 List of abbreviations .................................................................................................................... 12 Abstract ....................................................................................................................................... 14 Declaration ................................................................................................................................. -

Community Analysis of Plant Biomass-Degrading Microorganisms from Obsidian Pool, Yellowstone National Park
Microb Ecol DOI 10.1007/s00248-014-0500-8 ENVIRONMENTAL MICROBIOLOGY Community Analysis of Plant Biomass-Degrading Microorganisms from Obsidian Pool, Yellowstone National Park Tatiana A. Vishnivetskaya & Scott D. Hamilton-Brehm & Mircea Podar & Jennifer J. Mosher & Anthony V. Palumbo & Tommy J. Phelps & Martin Keller & James G. Elkins Received: 12 May 2014 /Accepted: 16 September 2014 # Springer Science+Business Media New York (outside the USA) 2014 Abstract The conversion of lignocellulosic biomass into (OBP), was examined for potential biomass-active microorgan- biofuels can potentially be improved by employing robust mi- isms using cultivation-independent and enrichment techniques. croorganisms and enzymes that efficiently deconstruct plant Analysis of 33,684 archaeal and 43,784 bacterial quality-filtered polysaccharides at elevated temperatures. Many of the geother- 16S rRNA gene pyrosequences revealed that archaeal diversity mal features of Yellowstone National Park (YNP) are surrounded in the main pool was higher than bacterial; however, in the by vegetation providing a source of allochthonic material to vegetated area, overall bacterial diversity was significantly support heterotrophic microbial communities adapted to utilize higher. Of notable interest was a flooded depression adjacent to plant biomass as a primary carbon and energy source. In this OBP supporting a stand of Juncus tweedyi, a heat-tolerant rush study, a well-known hot spring environment, Obsidian Pool commonly found growing near geothermal features in YNP. The microbial community from heated sediments surrounding the The submitted manuscript has been authored by a contractor of the U.S. plants was enriched in members of the Firmicutes including Government under contract DE-AC05-00OR22725. Accordingly, the potentially (hemi)cellulolytic bacteria from the genera U.S. -

Functional Comparison of Bacteria from the Human Gut and Closely
Functional comparison of bacteria from the human gut and closely related non-gut bacteria reveals the importance of conjugation and a paucity of motility and chemotaxis functions in the gut environment Dragana Dobrijevic, Anne-Laure Abraham, Alexandre Jamet, Emmanuelle Maguin, Maarten van de Guchte To cite this version: Dragana Dobrijevic, Anne-Laure Abraham, Alexandre Jamet, Emmanuelle Maguin, Maarten van de Guchte. Functional comparison of bacteria from the human gut and closely related non-gut bacte- ria reveals the importance of conjugation and a paucity of motility and chemotaxis functions in the gut environment. PLoS ONE, Public Library of Science, 2016, 11 (7), pp.e0159030. 10.1371/jour- nal.pone.0159030. hal-01353535 HAL Id: hal-01353535 https://hal.archives-ouvertes.fr/hal-01353535 Submitted on 11 Aug 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License RESEARCH ARTICLE Functional Comparison of Bacteria from the Human Gut and Closely Related Non-Gut Bacteria Reveals