Anaerobic Digestion of Biofuel Production Residues
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of High Cationic Flocculant on Anaerobic Digestion of Municipal Wastewater
University of Louisville ThinkIR: The University of Louisville's Institutional Repository Electronic Theses and Dissertations 5-2017 Effect of high cationic flocculant on anaerobic digestion of municipal wastewater. Prathap D. John University of Louisville Follow this and additional works at: https://ir.library.louisville.edu/etd Part of the Environmental Engineering Commons, and the Other Chemical Engineering Commons Recommended Citation John, Prathap D., "Effect of high cationic flocculant on anaerobic digestion of municipal wastewater." (2017). Electronic Theses and Dissertations. Paper 2815. https://doi.org/10.18297/etd/2815 This Master's Thesis is brought to you for free and open access by ThinkIR: The University of Louisville's Institutional Repository. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of ThinkIR: The University of Louisville's Institutional Repository. This title appears here courtesy of the author, who has retained all other copyrights. For more information, please contact [email protected]. EFFECT OF HIGH CATIONIC FLOCCULANT ON ANAEROBIC DIGESTION OF MUNICIPAL WASTEWATER By Prathap Daniel John B.S, University of Louisville, 2006 A Thesis Submitted to the Faculty of the University of Louisville J.B. Speed School of Engineering as Partial Fulfillment of the Requirements for the Professional Degree MASTER OF ENGINEERING Department of Chemical Engineering March 2017 ii EFFECT OF HIGH CATIONIC FLOCCULANT ON ANAEROBIC DIGESTION OF MUNICIPAL WASTEWATER APPROVAL PAGE Submitted by: __________________________________ Prathap Daniel John A Thesis Approved On ____________________________________ (Date) by the Following Reading and Examination Committee: ___________________________________ Dr. R. Eric Berson, Thesis Director ___________________________________ Dr. James Watters ___________________________________ Dr. Thomas Rockaway ii ACKNOWLEDGEMENTS I would like to thank the Conn Center for Renewable Energy Research and my research advisor Dr. -

Anaerobic Digestion of Primary Sewage Effluent
Anaerobic Digestion of Primary Sewage Effluent Prepared for the U.S. Department of Energy Office of Electricity Delivery and Energy Reliability Under Cooperative Agreement No. DE-EE0003507 Hawai‘i Energy Sustainability Program Subtask 3.4: Biomass Submitted by Hawai‘i Natural Energy Institute School of Ocean and Earth Science and Technology University of Hawai‘i September 2014 Acknowledgement: This material is based upon work supported by the United States Department of Energy under Cooperative Agreement Number DE-EE0003507. Disclaimer: This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference here in to any specific commercial product, process, or service by tradename, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. SUMMARY A hybrid system comprised an up-flow packed bed anaerobic reactor and a down-flow trickling-filter reactor connected in series was shown to effectively treat primary clarifier effluent. When a clarifier and sand filter were added to the system, the effluent water quality achieved values of BOD5 and TSS that were below the EPA’s water discharge limits of 30 mg/l and equivalent to highly efficient activated sludge systems. -

Heat Resistant Thermophilic Endospores in Cold Estuarine Sediments
Heat resistant thermophilic endospores in cold estuarine sediments Emma Bell Thesis submitted for the degree of Doctor of Philosophy School of Civil Engineering and Geosciences Faculty of Science, Agriculture and Engineering February 2016 Abstract Microbial biogeography explores the spatial and temporal distribution of microorganisms at multiple scales and is influenced by environmental selection and passive dispersal. Understanding the relative contribution of these factors can be challenging as their effects can be difficult to differentiate. Dormant thermophilic endospores in cold sediments offer a natural model for studies focusing on passive dispersal. Understanding distributions of these endospores is not confounded by the influence of environmental selection; rather their occurrence is due exclusively to passive transport. Sediment heating experiments were designed to investigate the dispersal histories of various thermophilic spore-forming Firmicutes in the River Tyne, a tidal estuary in North East England linking inland tributaries with the North Sea. Microcosm incubations at 50-80°C were monitored for sulfate reduction and enriched bacterial populations were characterised using denaturing gradient gel electrophoresis, functional gene clone libraries and high-throughput sequencing. The distribution of thermophilic endospores among different locations along the estuary was spatially variable, indicating that dispersal vectors originating in both warm terrestrial and marine habitats contribute to microbial diversity in estuarine and marine environments. In addition to their persistence in cold sediments, some endospores displayed a remarkable heat-resistance surviving multiple rounds of autoclaving. These extremely heat-resistant endospores are genetically similar to those detected in deep subsurface environments, including geothermal groundwater investigated from a nearby terrestrial borehole drilled to >1800 m depth with bottom temperatures in excess of 70°C. -

WO 2018/064165 A2 (.Pdf)
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/064165 A2 05 April 2018 (05.04.2018) W !P O PCT (51) International Patent Classification: Published: A61K 35/74 (20 15.0 1) C12N 1/21 (2006 .01) — without international search report and to be republished (21) International Application Number: upon receipt of that report (Rule 48.2(g)) PCT/US2017/053717 — with sequence listing part of description (Rule 5.2(a)) (22) International Filing Date: 27 September 2017 (27.09.2017) (25) Filing Language: English (26) Publication Langi English (30) Priority Data: 62/400,372 27 September 2016 (27.09.2016) US 62/508,885 19 May 2017 (19.05.2017) US 62/557,566 12 September 2017 (12.09.2017) US (71) Applicant: BOARD OF REGENTS, THE UNIVERSI¬ TY OF TEXAS SYSTEM [US/US]; 210 West 7th St., Austin, TX 78701 (US). (72) Inventors: WARGO, Jennifer; 1814 Bissonnet St., Hous ton, TX 77005 (US). GOPALAKRISHNAN, Vanch- eswaran; 7900 Cambridge, Apt. 10-lb, Houston, TX 77054 (US). (74) Agent: BYRD, Marshall, P.; Parker Highlander PLLC, 1120 S. Capital Of Texas Highway, Bldg. One, Suite 200, Austin, TX 78746 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Up Flow Anaerobic Filter (UAF) Treatment of Rural Domestic Sewage at Different Psychrophilic and Mesophilic Temperatures
2016 International Conference on Environment, Climate Change and Sustainable Development (ECCSD 2016) ISBN: 978-1-60595-358-8 Up Flow Anaerobic Filter (UAF) Treatment of Rural Domestic Sewage at Different Psychrophilic and Mesophilic Temperatures John Leju Celestino LADU 1,2 , Xi-wu LU 1,* and Zhong-zhao PING 1 1School of Energy and Environment, Department of Environmental Science and Engineering, Southeast University, Nanjing, 210096, P. R. China 2College of Natural Resources and Environmental Studies, Department of Environmental Studies, University of Juba, Republic of South Sudan *Corresponding author Keywords: Anaerobic filter, Pschrophilic, Mesophilic , Sewage, HRT. Abstract. This empirical research paper revealed the findings from the up flow anaerobic filter (UAF) treatment of rural domestic sewage at different pschrophilic and mesophilic temperature. The reactor was operated at specific HRT of 24, 48 and 72 hours. In the first 14 days of the start-up, the results obtained from the reactor revealed good removal efficiency. At the mesophilic temperature, average COD removal reached 82.0%; while at psychrophilic temperature the reactor attained 68.7%. From the overall results, UAF reactor managed under mesophilic condition disclosed higher COD removal efficiency as compared to psychrophilic temperature which is similar to values obtained in hot regions. Experimentally, up flow anaerobic filter reactor operated at mesophilic temperature is recommended for rural domestic sewage treatment at a specific HRT of 3 days. Introduction In recent years, gigantic urban areas are served more readily than the medium and small city areas or rural communities in domestic sewage treatment. Since the population density is less in rural areas as compared with big urban cities, a small-scale dispersed up flow anaerobic (UAF) treatment system is opted to be the best option. -

Innovations to Market
September 2013 SPECIAL FOCUS Featured Session Bringing Innovations to Market. Page 4 Featured Session Energy Generation in Fort Worth. Page 6 Innovation Showcase A Highlight of WEFTEC Programming. Page 8 Pavilion Exhibitors InnovatIon Innovation-award winning companies. Page 16 Innovation Showcase Visualize the potential of the water sector Actionable Water Market Intelligence ™ ® BlueTech Research, an O2 Environmental company, is an intelligence service focused exclusively on identifying key opportunities and emerging trends in the global water industry. We are the premier source of actionable BlueTech® Research offers analyst directed water market intelligence for strategic advisory services, providing market business decisions on innovative intelligence, technology assessments and technologies and companies. strategic advice. ® Our clients use BlueTech Research for: BlueTech® Intelligence Briefings ents. Snappy and informative,with distilled details prepared by BlueTech® Research water • Identifying and assessing water industry experts. companies and technologies BlueTech® Innovation Tracker™ • Understanding new water Innovation Company Tracker tool to map and analyse water technology market opportunities technology companies. and identifying areas for growth ® • Analyzing water technology patent BlueTech Insight Reports trends and identifying water Detailed reports providing insight and analysis on key water technology market areas. technology licensing, investment and acquisition opportunities BlueTech® Webinars Technology and Market -

Biogas Micro-Production from Human Organic Waste—A Research Proposal
sustainability Article Biogas Micro-Production from Human Organic Waste—A Research Proposal Alberto Regattieri * ID , Marco Bortolini ID , Emilio Ferrari, Mauro Gamberi and Francesco Piana Department of Industrial Engineering, Alma Mater Studiorum—University of Bologna, Viale del Risorgimento 2, Bologna 40136, Italy; [email protected] (M.B.); [email protected] (E.F.); [email protected] (M.G.); [email protected] (F.P.) * Correspondence: [email protected]; Tel.: +39-051-2093400 Received: 2 December 2017; Accepted: 24 January 2018; Published: 27 January 2018 Abstract: Organic waste (OW) management tackles the problem of sanitation and hygiene in developing countries and humanitarian camps where unmanaged waste often causes severe health problems and premature death. OW still has a usable energy content, allowing biogas production, potentially contributing to satisfy the local needs, e.g., cooking, lighting and heating. Digesters are the devices converting OW into biogas under anaerobic conditions. They are simple and effective solutions for the OW management in rural areas, humanitarian camps and remote developing regions, producing energy and fertilizers for local farming as residual. This paper describes the design and lab-test of a domestic OW management system integrating a waterless toilet with a small-scale digester producing safe biogas for local micro-consumption. Starting from people’s needs and an extensive review of the current state-of-art technology, the proposed system’s key innovation and strength is the combination of effectiveness and a very simple construction, set up and use, fitting with the rural conditions and raw materials available within the emerging countries. A small-scale prototype is assembled and lab-tested assessing the quantity—i.e., productivity—and quality—i.e., composition and methane content—of the produced biogas. -

Anaerobic Treatment of Municipal Wastewater
November 1984 ^ Env. Eng. Report No. 76-83-7 Anaerobic Treatment of Municipal Wastewater Michael S. Switzenbaum Associate Professor of Civil Engineering Kevin C. Sheehan Research Engineer and Robert F. Mickey Graduate Research Assistant The research upon which this report is based was supported by the Massachusetts Department of Environmental Quality Engineering, Division of Water Pollution Control, Contract No. 80-32. ENVIRONMENTAL ENGINEERING PROGRAM DEPARTMENT OF CIVIL ENGINEERING UNIVERSITY OF MASSACHUSETTS AMHERST, MASSACHUSETTS 01003 November, 1981) Env. Eng. Report No. 76-83-7 Technical Report Anaerobic Treatment of Municipal Wastewater by Michael S. Switzenbaum Assistant Professor of Civil Engineering Kevin C. Sheehan Research Engineer and Robert F. Hickey Graduate Research Assistant Department of Civil Engineering Environmental Engineering Program University of Massachusetts Amherst, MA 01003 Submitted to: Massachusetts Department of Environmental Quality Engineering Division of Water Pollution Control S. Russell Sylva, Commissioner Thomas C. McMahon, Director November 1984 ACKNOWLEDGEMENTS This study was supported by Research and Demonstration Programs from the Massachusetts Division of Water Pollution Control (Project Number 80-32). The authors would like to thank MDWPC for their support. In addition, the authors would like to thank Ecolotrol, Inc., for lending the pilot reactor system used in this study. Thanks are also extended to Ms. Candy Balmer, Messrs. Kurt Kallmeyer, Kevin Scheuer, and Michael Shapiro, graduate students in the Environmental Engineering Program at UMASS/Amherst for their assistance in the operation and analysis of the pilot reactor. In addition, thanks are extended to Mr. Stephen Plotkin, who was instrumental in setting up this project. Finally, special thanks are extended to Mrs. -
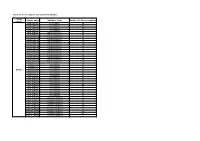
Table S1: List of Samples Included in the Analysis
Table S1: list of samples included in the analysis Study Sample name Inhibitory status Number of days at sampling number DNA.0P2T4 No inhibition 29 DNA.0P2T6 No inhibition 57 DNA.10P2T4 No inhibition 29 DNA.10P2T6 No inhibition 57 DNA.75P2T4 Phenol inhibition 29 DNA.75P2T6 Phenol inhibition 57 DNA.100P2T4 Phenol inhibition 29 DNA.100P2T6 Phenol inhibition 57 DNA.125P1T4 Phenol inhibition 29 DNA.125P1T6 Phenol inhibition 57 DNA.125P2T4 Phenol inhibition 29 DNA.125P2T6 Phenol inhibition 57 DNA.125P3T4 Phenol inhibition 29 DNA.125P3T6 Phenol inhibition 57 DNA.150P2T4 Phenol inhibition 29 DNA.150P2T6 Phenol inhibition 57 DNA.200P2T4 Phenol inhibition 29 DNA.200P2T6 Phenol inhibition 57 DNA.0N2T4 No inhibition 29 DNA.0N2T5 No inhibition 42 DNA.0N2T6 No inhibition 57 Study 1 DNA.5N2T4 No inhibition 29 DNA.5N2T5 No inhibition 42 DNA.5N2T6 No inhibition 57 DNA.10N2T4 No inhibition 29 DNA.10N2T5 No inhibition 42 DNA.10N2T6 No inhibition 57 DNA.15N2T4 No inhibition 29 DNA.15N2T5 No inhibition 42 DNA.15N2T6 No inhibition 57 DNA.25N2T4 No inhibition 29 DNA.25N2T5 No inhibition 42 DNA.25N2T6 No inhibition 57 DNA.75N2T4 Ammonia inhibition 29 DNA.75N2T5 Ammonia inhibition 42 DNA.75N2T6 Ammonia inhibition 57 DNA.100N2T4 Ammonia inhibition 29 DNA.100N2T5 Ammonia inhibition 42 DNA.100N2T6 Ammonia inhibition 57 DNA.250N2T4 Ammonia inhibition 29 DNA.250N2T5 Ammonia inhibition 42 DNA.250N2T6 Ammonia inhibition 57 nono2T3 No inhibition 16 noN2T4 Ammonia inhibition 23 noN2T8 Ammonia inhibition 60 noN2T9 Ammonia inhibition 85 noPhi2T4 Phenol inhibition 23 noPhi2T5 -

Anaerobic Treatment of Domestic Sewage In
Anaerobic treatment of domestic wastewater in subtropical regions Lucas Seghezzo Promotor: Prof. dr. ir. Gatze Lettinga, Hoogleraar in de anaërobe zuiveringstechnologie en hergebruik van afvalstoffen Co-promotoren: Dr. ir. Grietje Zeeman, Universitair docent bij de sectie Milieutechnologie Dr. Carlos Mario Cuevas, Universidad Nacional de Salta, Argentina Samenstelling promotiecommissie: Prof. dr. Henk Folmer (Wageningen Universiteit) Dr. ir. Ronald Mulder (Paques B.V., Nederland) Dr. ir. Bas van Vliet (Wageningen Universiteit) Prof. dr. Fernando Fernández-Polanco (Universidad de Valladolid, España) Dit onderzoek is uitgevoerd binnen de onderzoekschool WIMEK Anaerobic treatment of domestic wastewater in subtropical regions Lucas Seghezzo Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, prof. dr. ir. L. Speelman, in het openbaar te verdedigen op woensdag 26 mei 2004 des namiddags te vier uur in de Aula. CIP – DATA KONINKLIJKE BIBLIOTHEEK, DEN HAAG Author: Seghezzo, L. Title: Anaerobic treatment of domestic wastewater in subtropical regions Thesis Wageningen University, Wageningen, the Netherlands – with references – with summaries in English, Dutch, and Spanish. Publication year: 2004 ISBN: 90-8504-029-9 Subject headings: anaerobic treatment; domestic wastewater; sewage; UASB reactors; subtropical regions; sustainability assessment; sustainable development. Para Adriana, Natalia y Mateo “...que hay algunos que se cansan en saber y averiguar cosas que, una vez sabidas y averiguadas, no importan un ardite al entendimiento ni a la memoria...” (... because there are people who strive to know and find out things which, once known and found out, are not worth a straw to neither knowledge nor memory...) Don Quijote de la Mancha FOREWORD This thesis ends an era in my life and will hopefully open the doors of my future career. -

Overview of Anaerobic Digestion for Municipal Solid Waste
Global Methane Initiative Overview of Anaerobic Digestion for Municipal Solid Waste Updated: October 2016 1 About This Presentation . Introduces the process of anaerobic digestion (AD) for municipal solid waste (MSW) . Provides an overview of anaerobic digestion microbiology . Helps you understand how you might benefit from AD . Guides you through the key areas to consider when developing an AD project . Reviews the status of AD globally and provides selected case studies Using Bookmarks to Navigate This presentation contains bookmarks to help you navigate. Using the panel on the left, click the bookmark to jump to the slide. For Chrome users, the bookmarks can be viewed by clicking on the bookmark icon ( ) at the top right of the screen. 2 Global Methane Initiative GMI is a voluntary, multilateral partnership that aims to reduce global methane emissions and to advance the abatement, recovery and use of methane as a valuable clean energy source. OBJECTIVES BENEFITS . Reduce anthropogenic methane . Decline in methane concentrations emissions and advance the and methane utilization will result recovery and use of methane in: while: – Sustainability – Enhancing economic growth – Energy security – Promoting energy security – Health and safety – Improving local air quality – Profitability and public health. 3 GMI Partners . Grew from 14 to 42 Partner governments, plus the European Commission . Accounts for nearly 70% of global anthropogenic methane emissions 4 Main Menu 1. Introduction – what is AD and why should it interest me? Click here for an introduction to AD 2. Is AD suitable for me? Click here for more info about the potential for AD 3. Step-by-step guide Click here for detailed information about the key issues to consider when developing an AD project 4. -

Biomass Program Perspectives on Anaerobic Digestion and Fuel Cell Integration at Biorefineries
Biomass Program Perspectives on Anaerobic Digestion and Fuel Cell Integration at Biorefineries Biogas and Fuel Cell Brian Duff Workshop DOE Biomass Program NREL June 11,2012 1 | Biomass Program eere.energy.gov Outline • The Importance of Anaerobic Digestion for Fuels, Products, and Power • Biomass Program Perspective • The Potential for Biogas/Fuel Cell Integration at Biorefineries o Retrofit Applications for 1st-Generation Biofuels Plants o Integration Opportunities in Advance Biofuel Production • Potential for EERE Multi-Program Joint Solicitation 2 | Biomass Program eere.energy.gov Anaerobic Digestion: An Underappreciated and Underutilized Conversion Pathway for Biofuels, Bioproducts, and Bioenergy 3 | Biomass Program eere.energy.gov Anaerobic Digestion • Microbial bioconversion process generates methane • Emerging global technology • Has a “Bad Rep” in the US from numerous failed applications: still needs RD&D for widespread acceptance and commercialization • Reduces GHG emissions (22:1 vs. CO2) • Dependent on sales to grid like other distributed energy techs • Lack of national feed-in tariff retarding deployment • Lack of process reliability retarding financing and deployment 4 | Biomass Program eere.energy.gov Anaerobic Digestion Organic Recycling for Renewable Energy AD takes waste organic feedstocks… Into Renewable Energy… Municipal Operations Industrial And Recycles the Organics Electricity Operations Steam Institutions Correctional Organic Recycling Heat Homes and Renewable Pipeline Energy Facility Biogas Injection Restaurants