Contents Topic 1. Introduction to Microbiology. the Subject and Tasks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patterns, Processes and Models of Microbial Recovery in a Chronosequence Following Reforestation of Reclaimed Mine Soils
PATTERNS, PROCESSES AND MODELS OF MICROBIAL RECOVERY IN A CHRONOSEQUENCE FOLLOWING REFORESTATION OF RECLAIMED MINE SOILS Shan Sun Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Crop and Soil Environmental Sciences Brian Badgley, Chair Song Li Brian Strahm Michael Strickland Carl Zipper Virginia Polytechnic Institute and State University Blacksburg, Virginia July 2017 Keywords: soil microorganism, recovery, chronosequence, reclaimed mine soils, amplicon sequencing, shotgun metagenomics, bacterial co-occurrence groups, artificial neural network PATTERNS, PROCESSES AND MODELS OF MICROBIAL RECOVERY IN A CHRONOSEQUENCE FOLLOWING REFORESTATION OF RECLAIMED MINE SOILS Shan Sun Abstract Soil microbial communities mediate important ecological processes and play essential roles in biogeochemical cycling. Ecosystem disturbances such as surface mining significantly alter soil microbial communities, which could lead to changes or impairment of ecosystem functions. Reforestation procedures were designed to accelerate the reestablishment of plant community and the recovery of the forest ecosystem after reclamation. However, the microbial recovery during reforestation has not been well studied even though this information is essential for evaluating ecosystem restoration success. In addition, the similar starting conditions of mining sites of different ages facilitate a chronosequence approach for studying decades-long microbial community change, which could help generalize theories about ecosystem succession. In this study, the recovery of microbial communities in a chronosequence of reclaimed mine sites spanning 30 years post reforestation along with unmined reference sites was analyzed using next-generation sequencing to characterize soil-microbial abundance, richness, taxonomic composition, interaction patterns and functional genes. -

Download Download
http://wjst.wu.ac.th Natural Sciences Diversity Analysis of an Extremely Acidic Soil in a Layer of Coal Mine Detected the Occurrence of Rare Actinobacteria Megga Ratnasari PIKOLI1,*, Irawan SUGORO2 and Suharti3 1Department of Biology, Faculty of Science and Technology, Universitas Islam Negeri Syarif Hidayatullah Jakarta, Ciputat, Tangerang Selatan, Indonesia 2Center for Application of Technology of Isotope and Radiation, Badan Tenaga Nuklir Nasional, Jakarta Selatan, Indonesia 3Department of Chemistry, Faculty of Science and Computation, Universitas Pertamina, Simprug, Jakarta Selatan, Indonesia (*Corresponding author’s e-mail: [email protected], [email protected]) Received: 7 September 2017, Revised: 11 September 2018, Accepted: 29 October 2018 Abstract Studies that explore the diversity of microorganisms in unusual (extreme) environments have become more common. Our research aims to predict the diversity of bacteria that inhabit an extreme environment, a coal mine’s soil with pH of 2.93. Soil samples were collected from the soil at a depth of 12 meters from the surface, which is a clay layer adjacent to a coal seam in Tanjung Enim, South Sumatera, Indonesia. A culture-independent method, the polymerase chain reaction based denaturing gradient gel electrophoresis, was used to amplify the 16S rRNA gene to detect the viable-but-unculturable bacteria. Results showed that some OTUs that have never been found in the coal environment and which have phylogenetic relationships to the rare actinobacteria Actinomadura, Actinoallomurus, Actinospica, Streptacidiphilus, Aciditerrimonas, and Ferrimicrobium. Accordingly, the highly acidic soil in the coal mine is a source of rare actinobacteria that can be explored further to obtain bioactive compounds for the benefit of biotechnology. -

Successful Drug Discovery Informed by Actinobacterial Systematics
Successful Drug Discovery Informed by Actinobacterial Systematics Verrucosispora HPLC-DAD analysis of culture filtrate Structures of Abyssomicins Biological activity T DAD1, 7.382 (196 mAU,Up2) of 002-0101.D V. maris AB-18-032 mAU CH3 CH3 T extract H3C H3C Antibacterial activity (MIC): S. leeuwenhoekii C34 maris AB-18-032 175 mAU DAD1 A, Sig=210,10 150 C DAD1 B, Sig=230,10 O O DAD1 C, Sig=260,20 125 7 7 500 Rt 7.4 min DAD1 D, Sig=280,20 O O O O Growth inhibition of Gram-positive bacteria DAD1 , Sig=310,20 100 Abyssomicins DAD1 F, Sig=360,40 C 75 DAD1 G, Sig=435,40 Staphylococcus aureus (MRSA) 4 µg/ml DAD1 H, Sig=500,40 50 400 O O 25 O O Staphylococcus aureus (iVRSA) 13 µg/ml 0 CH CH3 300 400 500 nm 3 DAD1, 7.446 (300 mAU,Dn1) of 002-0101.D 300 mAU Mode of action: C HO atrop-C HO 250 atrop-C CH3 CH3 CH3 CH3 200 H C H C H C inhibitior of pABA biosynthesis 200 Rt 7.5 min H3C 3 3 3 Proximicin A Proximicin 150 HO O HO O O O O O O O O O A 100 O covalent binding to Cys263 of PabB 100 N 50 O O HO O O Sea of Japan B O O N O O (4-amino-4-deoxychorismate synthase) by 0 CH CH3 CH3 CH3 3 300 400 500 nm HO HO HO HO Michael addition -289 m 0 B D G H 2 4 6 8 10 12 14 16 min Newcastle Michael Goodfellow, School of Biology, University Newcastle University, Newcastle upon Tyne Atacama Desert In This Talk I will Consider: • Actinobacteria as a key group in the search for new therapeutic drugs. -
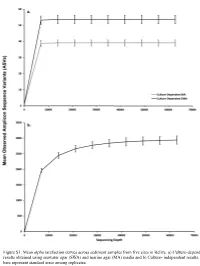
Figure S1. Mean Alpha Rarefaction Curves Across Sediment Samples from Five Sites in Belize
Figure S1. Mean alpha rarefaction curves across sediment samples from five sites in Belize. a) Culture-dependent results obtained using seawater agar (SWA) and marine agar (MA) media and b) Culture- independent results. Error bars represent standard error among replicates. a. 200 150 100 Faith’s PD Faith’s 50 0 Culturedependent MA Culturedependent SWA Cultureindependent Method b. 0.8 0.6 Pielou’s Evenness 0.4 Culturedependent MA Culturedependent SWA Cultureindependent Method Figure S2. Alpha diversity boxplots of marine sediment microbial communities from Carrie Bow Cay, Belize in culture-dependent and culture-independent samples determined using a) Faith’s Phylogenetic Diversity Index and b) Pielou’s Evenness. Culture-dependent methods include the use of marine agar medium (MA) and seawater agar medium (SWA. Data points are overlayed on the boxplot to show variation. a. 100% Proteobacteria Nitrospinae Bacteroidetes Fibrobacteres Planctomycetes Entotheonellaeota 90% Cyanobacteria Marinimicrobia (SAR406 clade) Firmicutes Deinococcus-Thermus Chloroflexi Margulisbacteria [A] Thaumarchaeota Unassigned 80% Acidobacteria Archaea UA Verrucomicrobia Acetothermia Actinobacteria Elusimicrobia 70% [A] Nanoarchaeaeota Tenericutes Kiritimatiellaeota TA06 Latescibacteria WS2 60% Spirochaetes Dependentiae Patescibacteria LCP-89 Gemmatimonadetes FCPU426 Omnitrophicaeota WPS-2 50% Fusobacteria CK-2C2-2 Bacteria UA Armatimonadetes [A] Euryarchaeota [A] Altiarchaeota Relative Percent 40% Calditrichaeota Poribacteria Lentisphaerae Cloacimonetes [A] Crenarchaeota -

MYB72-Dependent Coumarin Exudation Shapes Root Microbiome Assembly to Promote Plant Health
MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health Ioannis A. Stringlisa,1,KeYua,1, Kirstin Feussnerb,1, Ronnie de Jongea,c,d, Sietske Van Bentuma, Marcel C. Van Verka, Roeland L. Berendsena, Peter A. H. M. Bakkera, Ivo Feussnerb,e, and Corné M. J. Pietersea,2 aPlant–Microbe Interactions, Department of Biology, Science4Life, Utrecht University, 3508 TB Utrecht, The Netherlands; bDepartment of Plant Biochemistry, Albrecht-von-Haller-Institute for Plant Sciences, University of Göttingen, 37077 Göttingen, Germany; cDepartment of Plant Systems Biology, Vlaams Instituut voor Biotechnologie, 9052 Ghent, Belgium; dDepartment of Plant Biotechnology and Bioinformatics, Ghent University, 9052 Ghent, Belgium; and eDepartment of Plant Biochemistry, Göttingen Center for Molecular Biosciences, University of Göttingen, 37077 Göttingen, Germany Edited by Jeffery L. Dangl, University of North Carolina at Chapel Hill, Chapel Hill, NC, and approved April 3, 2018 (received for review December 22, 2017) Plant roots nurture a tremendous diversity of microbes via exudation of leaves do not display abundant transcriptional changes (9). photosynthetically fixed carbon sources. In turn, probiotic members of However, upon pathogen or insect attack, ISR-expressing leaves the root microbiome promote plant growth and protect the host plant develop an accelerated, primed defense response that is associated against pathogens and pests. In the Arabidopsis thaliana–Pseudomonas with enhanced resistance (9–11). In contrast to foliar tissues, simiae WCS417 model system the root-specific transcription factor WCS417-colonized roots show abundant transcriptional changes MYB72 and the MYB72-controlled β-glucosidase BGLU42 emerged as (9, 11–13). Among the WCS417-induced genes, the root-specific important regulators of beneficial rhizobacteria-induced systemic resis- R2R3-type MYB transcription factor gene MYB72 emerged as a tance (ISR) and iron-uptake responses. -

Supplementary Material 16S Rrna Clone Library
Kip et al. Biogeosciences (bg-2011-334) Supplementary Material 16S rRNA clone library To investigate the total bacterial community a clone library based on the 16S rRNA gene was performed of the pool Sphagnum mosses from Andorra peat, next to S. magellanicum some S. falcatulum was present in this pool and both these species were analysed. Both 16S clone libraries showed the presence of Alphaproteobacteria (17%), Verrucomicrobia (13%) and Gammaproteobacteria (2%) and since the distribution of bacterial genera among the two species was comparable an average was made. In total a 180 clones were sequenced and analyzed for the phylogenetic trees see Fig. A1 and A2 The 16S clone libraries showed a very diverse set of bacteria to be present inside or on Sphagnum mosses. Compared to other studies the microbial community in Sphagnum peat soils (Dedysh et al., 2006; Kulichevskaya et al., 2007a; Opelt and Berg, 2004) is comparable to the microbial community found here, inside and attached on the Sphagnum mosses of the Patagonian peatlands. Most of the clones showed sequence similarity to isolates or environmental samples originating from peat ecosystems, of which most of them originate from Siberian acidic peat bogs. This indicated that similar bacterial communities can be found in peatlands in the Northern and Southern hemisphere implying there is no big geographical difference in microbial diversity in peat bogs. Four out of five classes of Proteobacteria were present in the 16S rRNA clone library; Alfa-, Beta-, Gamma and Deltaproteobacteria. 42 % of the clones belonging to the Alphaproteobacteria showed a 96-97% to Acidophaera rubrifaciens, a member of the Rhodospirullales an acidophilic bacteriochlorophyll-producing bacterium isolated from acidic hotsprings and mine drainage (Hiraishi et al., 2000). -

Dissertation Implementing Organic Amendments To
DISSERTATION IMPLEMENTING ORGANIC AMENDMENTS TO ENHANCE MAIZE YIELD, SOIL MOISTURE, AND MICROBIAL NUTRIENT CYCLING IN TEMPERATE AGRICULTURE Submitted by Erika J. Foster Graduate Degree Program in Ecology In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Summer 2018 Doctoral Committee: Advisor: M. Francesca Cotrufo Louise Comas Charles Rhoades Matthew D. Wallenstein Copyright by Erika J. Foster 2018 All Rights Reserved i ABSTRACT IMPLEMENTING ORGANIC AMENDMENTS TO ENHANCE MAIZE YIELD, SOIL MOISTURE, AND MICROBIAL NUTRIENT CYCLING IN TEMPERATE AGRICULTURE To sustain agricultural production into the future, management should enhance natural biogeochemical cycling within the soil. Strategies to increase yield while reducing chemical fertilizer inputs and irrigation require robust research and development before widespread implementation. Current innovations in crop production use amendments such as manure and biochar charcoal to increase soil organic matter and improve soil structure, water, and nutrient content. Organic amendments also provide substrate and habitat for soil microorganisms that can play a key role cycling nutrients, improving nutrient availability for crops. Additional plant growth promoting bacteria can be incorporated into the soil as inocula to enhance soil nutrient cycling through mechanisms like phosphorus solubilization. Since microbial inoculation is highly effective under drought conditions, this technique pairs well in agricultural systems using limited irrigation to save water, particularly in semi-arid regions where climate change and population growth exacerbate water scarcity. The research in this dissertation examines synergistic techniques to reduce irrigation inputs, while building soil organic matter, and promoting natural microbial function to increase crop available nutrients. The research was conducted on conventional irrigated maize systems at the Agricultural Research Development and Education Center north of Fort Collins, CO. -

Using Community Analysis to Explore Bacterial Indicators for Disease
www.nature.com/scientificreports OPEN Using community analysis to explore bacterial indicators for disease suppression of tobacco Received: 08 February 2016 Accepted: 20 October 2016 bacterial wilt Published: 18 November 2016 Xiaojiao Liu, Shuting Zhang, Qipeng Jiang, Yani Bai, Guihua Shen, Shili Li & Wei Ding Although bacterial communities play important roles in the suppression of pathogenic diseases and crop production, little is known about the bacterial communities associated with bacterial wilt. Based on 16S rRNA gene sequencing, statistical analyses of microbial communities in disease-suppressive and disease-conducive soils from three districts during the vegetation period of tobacco showed that Proteobacteria was the dominant phylum, followed by Acidobacteria. Only samples from September were significantly correlated to disease factors. Fifteen indicators from taxa found in September (1 class, 2 orders, 3 families and 9 genera) were identified in the screen as being associated with disease suppression, and 10 of those were verified for potential disease suppression in March.Kaistobacter appeared to be the genus with the most potential for disease suppression. Elucidating microbially mediated natural disease suppression is fundamental to understanding microecosystem responses to sustainable farming and provides a possible approach for modeling disease-suppressive indicators. Here, using cluster analysis, MRPP testing, LEfSe and specific filters for a Venn diagram, we provide insight into identifying possible indicators of disease -

Diversity and Taxonomic Novelty of Actinobacteria Isolated from The
Diversity and taxonomic novelty of Actinobacteria isolated from the Atacama Desert and their potential to produce antibiotics Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Christian-Albrechts-Universität zu Kiel Vorgelegt von Alvaro S. Villalobos Kiel 2018 Referent: Prof. Dr. Johannes F. Imhoff Korreferent: Prof. Dr. Ute Hentschel Humeida Tag der mündlichen Prüfung: Zum Druck genehmigt: 03.12.2018 gez. Prof. Dr. Frank Kempken, Dekan Table of contents Summary .......................................................................................................................................... 1 Zusammenfassung ............................................................................................................................ 2 Introduction ...................................................................................................................................... 3 Geological and climatic background of Atacama Desert ............................................................. 3 Microbiology of Atacama Desert ................................................................................................. 5 Natural products from Atacama Desert ........................................................................................ 9 References .................................................................................................................................. 12 Aim of the thesis ........................................................................................................................... -

Flavobacterial Response to Organic Pollution
Vol. 51: 31–43, 2008 AQUATIC MICROBIAL ECOLOGY Published April 24 doi: 10.3354/ame01174 Aquat Microb Ecol Flavobacterial response to organic pollution Andrew Bissett1, 2, 3,*, John P. Bowman2, Chris M. Burke1 1School of Aquaculture, Tasmanian Aquaculture and Fisheries Institute, University of Tasmania and Aquafin CRC, Launceston, Tasmania 7250, Australia 2School of Agricultural Science, University of Tasmania, Hobart, Tasmania 7001, Australia 3Present address: Max Planck Institute for Marine Microbiology, Celsiusstrasse 1, 28359 Bremen, Germany ABSTRACT: Bacteria of the Cytophaga-Flavobacterium-Bacteroides (CFB) group (phylum Bac- teroidetes), in particular members of the class Flavobacteria, are among the most prominent hetero- trophic organisms in marine pelagic systems. They have also previously been found to be important in the initial biopolymer degradation of sedimentary organic matter. The Flavobacteria community was analysed in inshore, marine sediments subject to regular inputs of highly labile organic carbon in order to understand the importance of this group in carbon degradation. We used denaturing gra- dient gel electrophoresis and real-time PCR in a statistically robust manner, over 2 consecutive years, to demonstrate that the number of Flavobacteria in the sediment increased and community composi- tion shifted with organic loading. Further community shifts occurred after cessation of organic load- ing, and population numbers also decreased. Flavobacteria appear to be important in the initial responses of the sediment microbial community to organic loading, regardless of sediment type, but flavobacterial composition was not predictable. The highly dynamic nature and large diversity (func- tional redundancy) of the Flavobacteria in these sediments may contribute to this unpredictable response. KEY WORDS: Flavobacteria . -

Actinomycetes from the Coffee Plantation Soils of Western Ghats: Diversity and Enzymatic Potentials
Int.J.Curr.Microbiol.App.Sci (2018) 7(8): 3599-3611 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 7 Number 08 (2018) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2018.708.364 Actinomycetes from the Coffee Plantation Soils of Western Ghats: Diversity and Enzymatic Potentials Banu Sameera1, Harishchandra Sripathy Prakash2 and Monnanda Somaiah Nalini1* 1Department of Studies in Botany, 2Department of Studies in Biotechnology, University of Mysore, Manasagangotri, Mysore–570 006, Karnataka, India *Corresponding author ABSTRACT 230 soil actinomycetes were isolated from the coffee plantation of Western Ghats, Karnataka, India along the altitudinal gradients and depths. 24 morphologically distinct species were obtained based on the aerial spore chains and by the sequencing of the 16S K e yw or ds rRNA gene. The strains were assigned to the order Micrococcales, and novel orders Plantation soils, Pseudonocardiales ord. nov., Streptomycetales ord. nov., and Streptosporangiales ord. nov. Coffea arabica, The frequently isolated genus was Streptomyces, along with rare actinomycetes Streptomycetes, Actinomadura, Spirillospora, Actinocorallia, Arthrobacter, Saccharopolyspora and Rare actinomycetes, Nonomuraea. This study is the first report on Nonomuraea antimicrobica as a soil Soil properties, actinomycete. Diversity studies on the distribution of soil actinomycetes indicated enzymes significant differences (P< 0.05) among Shannon diversity indices of sample group depths Article Info along the slope. An attempt was made to correlate the total actinomycete count with soil parameters, by PCA based multiple linear regression (MLR) which significantly correlated Accepted: (P<0.0001) with pH, moisture, available nitrogen and phosphorous. About 91.6% of the 20 July 2018 isolates screened were found to be potentials for enzymatic activity. -

Marine Rare Actinomycetes: a Promising Source of Structurally Diverse and Unique Novel Natural Products
Review Marine Rare Actinomycetes: A Promising Source of Structurally Diverse and Unique Novel Natural Products Ramesh Subramani 1 and Detmer Sipkema 2,* 1 School of Biological and Chemical Sciences, Faculty of Science, Technology & Environment, The University of the South Pacific, Laucala Campus, Private Mail Bag, Suva, Republic of Fiji; [email protected] 2 Laboratory of Microbiology, Wageningen University & Research, Stippeneng 4, 6708 WE Wageningen, The Netherlands * Correspondence: [email protected]; Tel.: +31-317-483113 Received: 7 March 2019; Accepted: 23 April 2019; Published: 26 April 2019 Abstract: Rare actinomycetes are prolific in the marine environment; however, knowledge about their diversity, distribution and biochemistry is limited. Marine rare actinomycetes represent a rather untapped source of chemically diverse secondary metabolites and novel bioactive compounds. In this review, we aim to summarize the present knowledge on the isolation, diversity, distribution and natural product discovery of marine rare actinomycetes reported from mid-2013 to 2017. A total of 97 new species, representing 9 novel genera and belonging to 27 families of marine rare actinomycetes have been reported, with the highest numbers of novel isolates from the families Pseudonocardiaceae, Demequinaceae, Micromonosporaceae and Nocardioidaceae. Additionally, this study reviewed 167 new bioactive compounds produced by 58 different rare actinomycete species representing 24 genera. Most of the compounds produced by the marine rare actinomycetes present antibacterial, antifungal, antiparasitic, anticancer or antimalarial activities. The highest numbers of natural products were derived from the genera Nocardiopsis, Micromonospora, Salinispora and Pseudonocardia. Members of the genus Micromonospora were revealed to be the richest source of chemically diverse and unique bioactive natural products.