Analysis and Manufacturing of Cement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
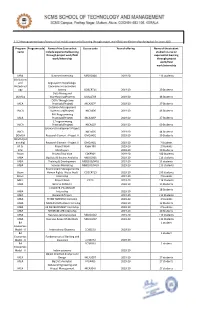
Experiential Learning- Projectwork- Fieldwork
1.3.2 Average percentage of courses that include experiential learning through project work/field work/internship during last five years (10) Program Program code Name of the Course that Course code Year of offering Name of the student name include experiential learning studied course on through project work/field experiential learning work/internship through project work/field work/internship MBA Summer Internship MB010304 2019-20 113 students BSc Botany and Angiosperm morphology, Biotechnol taxonomy and economic ogy botany BO6CRT11 2019-20 20 Students Data Mining and DDMCA Warehousing(Project) DMCA703 2019-20 48 Students OOPs Through Java MCA Practicals(Project) MCA307P 2019-20 27 Students Database Management IMCA Systems Lab(Project) IMCA406 2019-20 46 Students PHP Programmimg MCA Practicals(Project) MCA306P 2019-20 27 Students C Programmomg IMCA Practicals(Project) IMCA107 2019-20 59 Students SoGware Development-Project IMCA I IMCA605 2019-20 44 Students DDMCA Research Element - Project II DMCAX01 2019-20 39 Students DDMCA(Int ernship) Research Element - Project II DMCAX01 2019-20 7 Students M.Sc Project Work Paper XIII 2019-20 2 Students B.Sc MiniProject 2019-20 20 Students Bcom Project/Viva Voce C06PR01 2019-20 100 Students MBA Big Data & Busines Analytics MB010301 2019-20 113 students MBA Training & Development MB82 03/0401 2019-20 13 students MBA Services Marketing MB81 03/0403 2019-20 113 students Environment Management & Bcom Human Rights- Water Audit CO5CRT15 2019-20 100 Students Bcom Internship 2019-20 7 Students MBA Project Work -

Mumtaz Hotels Limited
MUMTAZ HOTELS LIMITED BOARD OF DIRECTORS Mr. Prithviraj Singh Oberoi, Chairperson Mr. Shivy Bhasin, Vice Chairman Mr. Bharath Bhushan Goyal, Managing Director (upto 6th April 2019) Mr. T. K. Sibal Mr. Manish Goyal, Managing Director (w.e.f. 17th May 2019) Mr. Vikramjit Singh Oberoi Mr. Arjun Singh Oberoi Mr. Manav Goyal (w.e.f. 17th May 2019) Mr. Raj Kumar Kataria, Independent Director (upto 25th February 2020) Additional Director (w.e.f. 31st March 2020) Mr. Sandeep Kumar Barasia, Independent Director Dr. Chhavi Rajawat, Additional Director (w.e.f. 25th October 2019) CHIEF FINANCIAL OFFICER Mr. Kallol Kundu SECRETARY Mr. S.N. Sridhar AUDITORS Deloitte Haskins & Sells LLP, Chartered Accountants 7th Floor, Building 10, Tower B DLF Cyber City Complex DLF City Phase – II Gurugram – 122002 Haryana REGISTERED OFFICE 4, Mangoe Lane Kolkata 700 001 CORPORATE OFFICE 7, Sham Nath Marg Delhi 110 054 DIRECTORS’ REPORT The Members Mumtaz Hotels Limited The Board presents its Thirtieth Annual Report together with the Audited Financial Statement and the Auditor’s Report in respect of the Financial Year ended on 31st March 2020. Financial Highlights The Financial Highlights of the year under review as compared to the previous year are given below: PARTICULARS ` (in million) 2019-20 2018-19 Total Revenue 1005.42 1,071.29 Earnings before Interest, Depreciation and Amortization, Taxes 436.39 496.38 and Exceptional Items (EBIDTA) Finance Costs 0.18 0.32 Depreciation 28.12 22.34 Profit before Tax 408.09 473.72 Current Tax 105.12 135.47 Deferred Tax (18.46) 0.53 Profit after Tax 321.43 337.72 Other Comprehensive Income/(Loss), net of tax (0.47) (0.06) Total Comprehensive Income 320.96 337.66 Profit/ (Loss) Brought forward from earlier years 777.14 626.20 Dividend (154.88) (154.88) Dividend Distribution Tax (31.84) (31.84) General Reserve - - Profit/ Loss Carried Over 911.38 777.14 Performance During the Financial Year under review, the Company’s Total Revenue was ` 1005.42 million as compared to ` 1,071.29 million in the previous year. -

Malabar Cements Limited
MALABAR CEMENTS LIMITED CORPORATE SOCIAL RESPONSIBILITY POLICY 1. Preamble Corporate Social Responsibility is a voluntary concept that consists of environmental and social issues with the aim to improve community well-being, respect human rights and to preserve the environment. CSR is also closely linked to the concept of Sustainable Development. The objectives of business should be of three fold, namely profit, people and planet. Business must sub serve the economic, social and the environmental concerns in its growth strategy. This CSR policy will form as a master guide to the implementation of social responsibility measures of Malabar Cements Limited. The Companies Act, 2013 has introduced the idea of CSR to the forefront and through its disclose-or-explain mandate, promoting greater transparency and disclosure to the CSR activities of the Company. Further the Companies (CSR Policy) Rules, 2014 lays down the framework and modalities of carrying out CSR activities, which are specified in, schedule VII of the act. 2. CSR Outlook Malabar Cements Limited is one of the prestigious organizations of the Government of Kerala in Palakkad District. This Company incorporated in the year 1978 is the only integrated grey cement manufacturing plant in Kerala with its captive Limestone mine at Walayar. MCL is a healthy Company, with sound financial base. Company started earning Net profit continuously from the year 1991-92 onwards. Even before the introduction of Company Act 2013, MCL utilized a share of its profit for CSR activities. Many social welfare projects in this regard were implemented from 2012 onwards. In association with Kerala State Security Mission many CSR continuing schemes are implemented. -

Public Sector Under Takings
GOVERNMENT OF KERALA KERALA STATE PLANNING BOARD THIRTEENTH FIVE-YEAR PLAN (2017-2022) WORKING GROUP ON PUBLIC SECTOR UNDER TAKINGS REPORT INDUSTRY AND INFRASTRUCTURE DIVISION KERALA STATE PLANNING BOARD THIRUVANANTHAPURAM MARCH 2017 PREFACE In Kerala, the process of a Five-Year Plan is an exercise in people’s participation. At the end of September 2016, the Kerala State Planning Board began an effort to conduct the widest possible consultations before formulating the Plan. The Planning Board formed 43 Working Groups, with a total of more than 700 members – scholars, administrators, social and political activists and other experts. Although the Reports do not represent the official position of the Government of Kerala, their content will help in the formulation of the Thirteenth Five-Year Plan document. This document is the report of the Working Group on Public Sector Enterprises. The Chairpersons of the Working Group were Sri. Paul Antony IAS, Additional Chief Secretary, Industries Department and Dr. M.P. Sukumaran Nair, Chairman, RIAB. The Member of the Planning Board who coordinated the activities of the Working Group was Dr. Jayan Jose Thomas. The concerned Chief of Division was Sri. Joy N.R. Member Secretary FOREWORD Industrial development is crucial for the growth of any nation. It is also linked to the modernization of agriculture, development of science and technology, entrepreneurship, self- reliance in defense production, success in international trade, efficient utilization of natural resources, alleviation of poverty and unemployment and increase in per capita income and standard of living of the people. Expansion of industry and Services is essential for economic development and growth, as these are major enablers of productivity increases. -

MALABAR CEMENTS LIMITED (A Govt
MALABAR CEMENTS LIMITED (A Govt. of Kerala Undertaking) WALAYAR, PALAKKAD DISTRICT, KERALA-678 624 Ph: 2862266/73/74 Fax: 0491-2862230 Website: www.malabarcements.com E.mail:[email protected] NOTICE INVITING TENDER Tender No. MCL/02/PRT/830/2020 Date.05.05.2020 Separate tenders are being invited by the Managing Director, on behalf of the Malabar Cements Ltd,Walayar(P.O) Palakkad 678624,Kerala India, through electronic tender (e-tender) for the Works/Service mentioned in the list given in next page from eligible and resourceful bidders having sufficient credential and financial capability for execution of Works /Service as per the tender. Intending bidders desirous of participating in the e-tender are to login to the website www.etenders.kerala.gov.in (the official website of kerala Government e portal) Contractors/bidders willing to take part in the process of e-tender are required to obtain Digital Signature Certificate (DSC) from any of the authorized ‘Certifying Authorities’ (CA) The prospective bidders may contact the e-tendering State Level Help desk located Thiruvananthapuram Ph: 0471 2577088, Kannur Ph: 0497 2764188 , 0497 2764788 and Malappuram Ph: 0483 7732941 on any working day within working hours for any query on e-tendering. Intending bidders are required to download the e-tender documents directly from the website www.etenders.kerala.gov.in . Tender documents consisting of Technical specification, schedule of quantities, PAGE NO. 1 commercial terms and conditions etc. MCL reserves the right to reject any or all tenders without assigning any reason thereof. If any clarification required, tenderers may also send their queries to the e-mail [email protected] . -

O4jcr3f,O Cs4 4
13-Oo O60F (T,lQrocue olo)lo(l)cmco (ruo4og(I)o magto !.n6rpo1so6np oaloBro mo:567 10.08.2014-d oo)olslctdd, OoJC@COOlelC (tu65^lmaEn6tf,rc r,r^r'llm6- o4Jcr3f,o gGnDoo l&1."Oo. .oomJ [@1. o-n.o6. 6]durooen6o]g,l ((UjoJfiuocor(U)o ojloJo fluoob(D'l6oJlo clet*J ornol) (.O) (ru.mmc.n(o|o" oO@ 6)drcpoo6lelc (€) 2013-14 aoeJ@eloird d,mrrlaEld (rtcna" o{o(rDc(o) o\rl}cdl(r)arBoa el.eo@oc(ot er44 o|onrl{lco qc6"g@ @oiaoro)cD @or6(dE@(mo6rB(rro.(sooo{]o(oqco rdlootoelc6(T)[email protected]. .OCrOo O$dlOOC6)CC@c; (TUomtOCCnOlOllt o5otm)cOr Oa.dlOr$ croutcDo(otqee 15 o.rcpco6lelc cDocdloarBec6m- e]cBolold @odorDl4(U): eicc(dotd @ol(a(drdl4 m)ocoJo@Eq6s olcx.do Grd(o6Dtou)o 6t(mc(on q-td@|otadlefilo. (srjl) 0101.2011 oer 6mD6" @acoo o4o.o)sco (5nl) 2O1Oll ducn!@djle o6.sooro 6,5rD66,oa o.rcp(Da|elcoJ.llmjca,(lloonrrr@Bo8 etocooaccoroil(i@od@ot4o.,rcpcooreo ercG6@occor@,od(orol-drooo:ojlcoocorno o{crcuc(o .y\)ocorfi)eBgosoLx@. elGlocdccoc; GrocF6tufitDo 66rBCCDl Odd(t'roJld6(ro, (ad) (n.E(oroid @o6oril4 orcrm (.n ) 2010-11 (rucfillrorole otd.so(oto 65rD€6sA 6k1lcprlo6leDo{ormiceo)mlocdJ(n6t3ua @ac(do(r)qoiotd@ol6(od4 .6l6xo9loo; @rru(o cruocoJoeBo€ ooJcpco6)tac ffuocorn@Bgos olo(do elcl36@oc6fi)ot(r)cc{@ doooloxd Grdco6nicr\l). qcmcst cdrEoroJlolacrn. orco6s-(r)sqjleJc6cm(otfil(r)soJdld,d m.ry@loJlad@o6(o|6t6(rDodrcgcootelc arulla(o16ooc;ol(6rjc.(6" eJBlocaccoc; (ruocdlodrBogoaoiloggoojl elcc6(.OC6fin(6t(r)c(D, .id"e/ hgfu4cel dcD.diloua Gqot.gdaf nud@c(dl6r0 mrccnroojla fu.ocaoconrcos (r)sqjleloddl o(acrE, Cs4 4- oald,graa 6]cdnlaud GFm6 mDo - | Sl No Name of Company KERALA STATE INDUSTRIAL DEVELOPMENT ]. -

Government Of'kerala
GOVERNMENT OF'KERALA F'INANCE (BUDGET WING. A) DEPARTMENT CIRCULAR No.09/2021lX'in. Dated, Thiruvananthapuram, 27th J anrtary 2021. Sub:- Budget 2021-22 - Budget documents - Distribution to the Heads of Departments - Instructions - Issued. Arrangements have been made for the printing and supply of the Budget documents for 2021-2022 to all concerned. The Superintendent of Government Presses, Thiruvananthapuram will distribute the documents to the Heads of Departments and others in accordance *ith the fixed scale of supply. He will also distribute one set each containing all documents to the Public Sector Undertakings. All Heads of Departments/Public Sector Undertakings are therefore directed to collect the copies of budget documents 2O2l-22 from the Government Press, Thiruvananthapuram. The Heads of Departments will note that though there are three volumes of Demands for Grants, they will be supplied with the volumes concemed with their Deparffnents outlay only. One set of documents for the purpose of distribution will consist of the following items. l. Demands for Grants (Relevant Volumes only) 2. Revenue Budget 3. Explanatory Memorandum 4. Works Appendix-Vol.I ,. 5. Debt Head Budget 6. Budget Speech (English & Malayalam) 7. Appendix IV (Provisions earmarked to Local Self Government Institutions) 8. Thirteenth Five Year Plart 2017- 2022 - 5ft Year's Programm e - 2021-22 9. - Annual Plan202l-22 10. Vote on Account In addition to the items mentioned above, all Heads of Departments and others would be supplied with one copy each of the following documents th-at are meant for restricted Q supply. 1. Appendix I (Details of staff) 2. Annual Financial Statement 3. -
Table 8 Performance of Public Enterprises in Kerala
Table 8 Performance of Public Enterprises in Kerala Sl. Table Page No 1 An Overview of Performance of Public Enterprises in Kerala (Government 2 Companies) 2 An Overview of Performance of Public Enterprises in Kerala (Statutory Bodies) 3 3 Sector wise Summary of Performance of Public Enterprises in Kerala 2015-16 4 4 Enterprises Merged, Transferred, Closed, Taken Over, Liquidated or Inactive 5 5 Sector wise Classification of State Level Public Enterprises (SLPEs) 6 6 Status of Ownership of State Level Public Enterprises (SLPEs) 7 7 Administrative Departments of the State Level Public Enterprises (SLPEs) 8 8 Arrears in Audit of Accounts 9 9 Overall Performance of State Level Public Enterprises (SLPEs) in Kerala : 10 2013-14 to 2015-16 10 Top Ten Enterprises in terms of Capital Invested during 2015-16 11 11 Top Ten Enterprises in terms of Turnover during 2015-16 12 12 Top Ten Profit making Enterprises during 2015-16 13 13 Top Ten Loss Incurring Enterprises during 2015-16 14 14 Top Ten Enterprises in terms of Employment 15 15 Top Ten Negative Net Worth Enterprises during 2015-16 16 16 Top Ten Enterprises in terms of Contribution to State Exchequer 17 17 List of Enterprises with Accumulated Loss in Kerala 18 1 Table 8.1 An Overview of Performance of Public Enterprises in Kerala (Government Companies) (Amount in C Crore) Year No. of Total Paid up Capital Units on Profit Unit on Loss Net Dividend Declared/ Units Employment Capital Invested Profit/Loss Proposed No. of Amount No. of Amount (+/-) No. of Amount Units Units Units 2006-07 96 50361 -

And Activities Incidental to Mining Operations Is an Exempted Activity from Down
-\ Government of Kerala Abstract GAD-Covid-Ig general lock down- - exemption categories-permission for mineral based industries- orders issued General Administration (Secret Section) Department Go (Ms) No. 60I2020/GAD Thiruvananthapur am dated, 04 /04 /2020 Read: 1. GO (M) a9/2020/GAD dated 2g.02.2020 2' Order no.40-3/2020-DM-I(A) dated 24.09.2020,25.03.2020 and 02.04.2020 of MHA, Government of India ORDER Central and state Governments have enJorced regulations in the form of Lock Down to contain the spread of Novel Corona virus (Covid-19). As per the addendum dated 25'03.2020 to the general lock down ora", issuea uy Ministry of Home Affairs coal and mineral production, transportatiory supply of explosives and activities incidental to mining operations is an exempted activity from down. lock Hence' Government are pleased to permit the functioning of following public units and all private sector units in this sector subject to the strict adherence of conditions laid down in the guiderines issued by Ministry of Home Affairs: i' Kerala Minerars and Metars Limited, Chavara ii. Indian Rare Earths Limited, Chavara iii. Travancore products Titanium Limited, Kochu veri iv. Travancore Cements limited, Kottayam v. Malabar Cements Limited, palakkad since Travancore Cochin Chemicals Limited, Kalamass eri, aKerala psu, is the lone unit which manufacfures and supplies Hydrochloric Acid, a main raw material required for the above productio.r rritr, permission is also granted for the functioning of Travancore Cochin Chemicals Limited subject to the strict -

MALABAR CEMENTS LIMITED (A Govt
MALABAR CEMENTS LIMITED (A Govt. of Kerala Undertaking) WALAYAR, PALAKKAD DISTRICT, KERALA-678 624 Ph: 2862266/73/74 Fax: 0491-2862230 Website: www.malabarcements.com E.mail: [email protected] Tender No. MCL/02/PRT/676/2013 Date: 30.04.2013 CLEARING & FORWARDING OF CLINKER FROM PIRAVOM ROAD TO OUR CEMENT GRINDING UNIT AT PALLIPURAM, CHERTHALA, ALLEPPEY (DIST) TENDER DOCUMENT 1.GENERAL INSTRUCTIONS a. The tender is invited for and on behalf of the Managing Director, Malabar Cements Limited, Walayar.PO, Palakkad 678624, Kerala, India for clearing of Clinker from the railway wagons at PIRAVOM ROAD siding and transport the same by tippers/trucks to our Cement Grinding Unit at Pallipuram Cherthala ,Alleppey Dist b. Any offer made in response to this tender, when accepted by Malabar Cements Limited (MCL), Walayar, Palakkad will constitute a contract between the parties by issuing a Work Order. c. The tenderer shall remit an amount of Rs.26,250/-(Rupees twenty six thousand two hundred fifty Only) by online payment SBT & SBT NEFT towards the cost of tender documents.Scanned copy of online payment documents to be enclosed along with the tender. II.SUBMISSION OF TENDER: a. Every tender shall be made in English. Tender should be free from overwriting. The tenderer should duly attest all corrections and alterations. b. Tender is in 2 cover system, ie, cover I (Technical & Commercial Bid) and cover II (Price bid). In the cover I of the tender, the Tenderer should give all the required general conditions with supporting documents as per Tender Signature of the tenderer with seal 1 conditions and Commercial Terms and in cover-II Price Bid, only price to be quoted and the tender’s name in the relevant column c. -

Malabar Cements Limited
Rating Advisory March 01, 2019 | Mumbai Adarsh BirmechaOri ginal Template123 Malabar Cements Limited Advisory as on March 01, 2019 This rating advisory is provided in relation to the rating of Malabar Cements Limited The key rating sensitivity factors for the rating include: Significant debt-funded capital expenditure (capex) plans Sustenance of growth and profitability Working capital management CRISIL Ratings has a policy of keeping its accepted ratings under constant and ongoing monitoring and review. Accordingly, it seeks regular updates from companies on business and financial performance. CRISIL is yet to receive adequate information from Malabar Cements Ltd (MCL) to enable it to undertake a rating review. CRISIL is taking all possible efforts to get the rated entity to cooperate with its rating process for enabling it to carry out the rating review. CRISIL views information availability risk as a key factor in its assessment of credit risk. (Please refer to CRISIL Ratings publication dated April 30, 2012 - 'Information Availability - a key risk factor in credit ratings') If MCL continues to delay the provisioning of information required by CRISIL to undertake a rating review then, in accordance with circular SEBI/HO/MIRSD/MIRSD4/CIR/P/2016/119 dt Nov 1, 2016 and SEBI/HO/MIRSD/ MIRSD4/ CIR/ P/ 2017/ 71 dt June 30, 2017 issued by Securities and Exchange Board of India, CRISIL will carry out the review based on best available information and issue a press release. About the Company MCL, which commenced operations in 1984, manufactures Portland Pozzolana, Portland slag cement and specialty cement. The company operates two cement plants with capacity of 8.6 lakh tonnes per annum at Walayar, and Cherthala (Kerala) and has an established market position across Kerala. -

Committee Public Undertakings
FOURTIBBNTH KFRALA LECISLATTVE ASSEMBLY COMMITTEE ON PUBLIC UNDERTAKINGS (2016-2Or9) SBVENTY FIFTH REPORT (Presented on 4th December, 2018) SECRETARTAT OF THE KERALA LEGISLATURE THIRIJVANANTHAPURAM 2018 FOURTEENTH KERALA LEGISLATIVE ASSEMBLY COMMITTEE ON PUBLIC UNDERTAKINGS (2016-2Or9) AR CEMENTS LIMITED (Bared oa the Rcport of tto Cornptrollq and Auditor Gcncral of Iadie for thc yca,r oldcd 3l March' 20lt 6U2019. CONTENTS Page Composition of the Committee Introduction .. vii Report .. I Appendix I j Summary of main Conclusions/Recommendations 10 Appendix tr : Notes fumished by Government on th€ Audit Paragraph CoMMTTTEE ON PUBLTC LJNDERTAKTNGS (20162019) COMPOSITION OT ITTS COMMITTEE Chaitman : Shri C. Divakaran. Members : Shri T. A. Ahammed Kabeer . Shri K. B. Ganesh Kumar Shri C. Krishnan Shri S. Rajendran Shri Thiruvanchoor Radhakrishnan Shri P. T. A. Rahim Shri Raju Abraham Shri Sunny Joseph Shri C. F. Thomas Shri P. Unni. Legislature Secrewiat : Shri V. K. Babu Prakash, Secretary Shri K. Suresh Kumar,.Joint Secretary Shri G. Harish, Deputy Secretary Smt. Deepa V., Under Secretary. INTRODUCTION I, the Chairman, Committee on Public Undertakings (201G2019) having been authorised by the Committee to prcsent the Report on their behalf, present this Seventy Fifth Report on Malabar Cements Limited based on the Report of the Comptroller and Auditor General of India'for the year ended 3l March, 2015 relating to the Pubtic Sector Undertakings of the Government of Kerala. The aforesaid Report of the Comptroller and Auditor General of India for the year ended 3l March, 2015 was laid on the Table of the House on 2&62016. The consideration of the audit paragraphs included in this Report and the examination of the departrnental witness in connection thereto was made by the Committee on Public Undertakings constituted for the years 201G2019 at its meeting held on l7-1U2017.