Understanding the Origin of the Vertebrates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review Sheet for Lecture 15: Life in the Benthic Realm
Lecture 15: Life in the Benthic Realm Terminology Planktonic, nektonic, benthic, epifaunal, infaunal, sessile, mobile, bathyal, abyssal, abyssopelagic, hadopelagic, supratidal, intertidal, subtidal, spicules, spongin, radial symmetry, encruster, epidermal cell, porocyte, sclerocyte, amoebocyte, mesohyl, choanocyte, polyp, medusa, colonial, solitary, aragonite, octocoral, gorgonin, cnidocyst, nematocyst, zooxanthellae, hematypic, coral bleaching, lophophore, zoid, zooecium, zooaria, polymorphism, pedicle, helically coiled shell, radially coiled shell, exoskeleton, endoskeleton Dates: none Review Questions: Be familiar with the life and feeding modes of all of the invertebrate groups we discussed and their major morphologies, behaviors, life strategies etc. Why are so many sessile invertebrates radially symmetric? Explain the three different wall types found in sponges, what’s the point? Discuss the different roles of the different sponge cells How do hematypic corals contribute to the creation of different nearshore environments and conditions? Describe the community created by an innkeeper worm How does the skeleton of a brachiopod differ from that of a clam? How do the shells of clams, snails, and nautiloids differ? Classification: Phylum Porifera Class Demospongiae (soft sponges) Class Calcarea (calcareous sponges) Class Hexactinellida (glass sponges) Phylum Cnidaria Class Anthozoa -stony corals -sea fans -soft corals -sea anemones -sea pens Phylum Annelida (worms) -bristleworms -innkeeper worm -lugworms -spaghetti worm -feather duster -catworm Phylum Bryozoa (moss-animals) Phylum Mollusca -Class Bivalvia (clams) -Class Gastropoda (snails and slugs) -Class Cephalopoda (squid, octopus, nautiloid) -Class Scaphopoda (tusk shells) -Class Polyplacophora (chitons) . -

Invertebrates Invertebrates: • Are Animals Without Backbones • Represent 95% of the Animal Kingdom Animal Diversity Morphological Vs
Invertebrates Invertebrates: • Are animals without backbones • Represent 95% of the animal kingdom Animal Diversity Morphological vs. Molecular Character Phylogeny? A tree is a hypothesis supported or not supported by evidence. Groupings change as new evidence become available. Sponges - Porifera Natural Bath Sponges – over-collected, now uncommon Sponges • Perhaps oldest animal phylum (Ctenphora possibly older) • may represent several old phyla, some now extinct ----------------Ctenophora? Sponges - Porifera • Mostly marine • Sessile animals • Lack true tissues; • Have only a few cell types, cells kind of independent • Most have no symmetry • Body resembles a sac perforated with holes, system of canals. • Strengthened by fibers of spongin, spicules Sponges have a variety of shapes Sponges Pores Choanocyte Amoebocyte (feeding cell) Skeletal Water fiber flow Central cavity Flagella Choanocyte in contact with an amoebocyte Sponges - Porifera • Sessile filter feeder • No mouth • Sac-like body, perforated by pores. • Interior lined by flagellated cells (choanocytes). Flagellated collar cells generate a current, draw water through the walls of the sponge where food is collected. • Amoeboid cells move around in the mesophyll and distribute food. Sponges - Porifera Grantia x.s. Sponge Reproduction Asexual reproduction • Fragmentation or by budding. • Sponges are capable of regeneration, growth of a whole from a small part. Sexual reproduction • Hermaphrodites, produce both eggs and sperm • Eggs and sperm released into the central cavity • Produces -

S I Section 4
3/31/2011 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Porifera Ecdysozoa Deuterostomia Lophotrochozoa Cnidaria and Ctenophora Cnidaria and Protostomia SSiection 4 Radiata Bilateria Professor Donald McFarlane Parazoa Eumetazoa Lecture 13 Invertebrates: Parazoa, Radiata, and Lophotrochozoa Ancestral colonial choanoflagellate 2 Traditional classification based on Parazoa – Phylum Porifera body plans Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Parazoa 4 main morphological and developmental Sponges features used Loosely organized and lack Porifera Ecdysozoa Cnidaria and Ctenophora tissues euterostomia photrochozoa D 1. o Presence or absence of different tissue L Multicellular with several types of types Protostomia cells 2. Type of body symmetry Radiata Bilateria 8,000 species, mostly marine Parazoa Eumetazoa 3. Presence or absence of a true body No apparent symmetry cavity Ancestral colonial Adults sessile, larvae free- choanoflagellate 4. Patterns of embryonic development swimming 3 4 1 3/31/2011 Water drawn through pores (ostia) into spongocoel Flows out through osculum Reproduce Choanocytes line spongocoel Sexually Most hermapppgggphrodites producing eggs and sperm Trap and eat small particles and plankton Gametes are derived from amoebocytes or Mesohyl between choanocytes and choanocytes epithelial cells Asexually Amoebocytes absorb food from choanocytes, Small fragment or bud may detach and form a new digest it, and carry -

Practical Paleoecology GEO 342
Practical Paleoecology GEO 342 Compiled By: Dr. Osama Attia Compiled By Dr. Osama Attia 1 Phylum Brachiopoda Brachiopods or lamp shells Name: Name means "arm" (brachio) + "foot" (pod). Main characteristics: - Bivalved (two shells), each with bilateral symmetry. - The plane of symmetry passes through the center of each shell or valve. - The two valves differ in size and shape in most. Sometimes the larger valve will have an opening near the hinge line through which the pedicle extended in life. - Soft parts include a lophophore consisting of coiled tentacles with cilia. The lophophore circulates water between the two valves, distributing oxygen and flushing out carbon dioxide. Water movements caused by the lophophore also transport food particles toward the mouth. Geologic range: Lower Cambrian to Recent. Very abundant during the Paleozoic. A few species (belonging to only three families) remain today. Mode of life: Inhabitants of shallow marine environments; they generally live attached in a fixed position on the seafloor. Inarticulate brachiopods are known to live in burrows in the sediment. Brachiopods are filter feeders. Compiled By Dr. Osama Attia 2 Living positions of brachiopods. A = Articulate brachiopod attached to the seafloor by its pedicle. B = Interior of brachiopod valve showing lophophore. C = Inarticulate brachiopod, Lingula, which lives within a tube or burrow in seafloor sediment. A. CLASS INARTICULATA – The Inarticulate Brachiopods Primitive brachiopods with phosphatic or chitinous valves; no hinge. Spoon-shaped valves held together with muscles and soft parts. Lingula is a well known inarticulate brachiopod. Geologic range: Lower Cambrian to Recent Compiled By Dr. Osama Attia 3 Fossil inarticulate brachiopod from the Cambrian. -

Animal Diversity Part 2
Textbook resources • pp. 517-522 • pp. 527-8 Animal Diversity • p. 530 part 2 • pp. 531-2 Clicker question In protostomes A. The blastopore becomes the mouth. B. The blastopore becomes the anus. C. Development involves indeterminate cleavage. D. B and C Fig. 25.2 Phylogeny to know (1). Symmetry Critical innovations to insert: Oral bilateral symmetry ecdysis mouth develops after anus multicellularity Aboral tissues 1 Animal diversity, part 2 Parazoa Diversity 2 I. Parazoa • Porifera: Sponges II. Cnidaria & Ctenophora • Tissues • Symmetry I. Outline the • Germ Layers III. Lophotrochozoa unique • Embryonic characteristics Development of sponges IV. Ecdysozoa • Body Cavities • Segmentation Parazoa Parazoa • Porifera: Sponges • Porifera: Sponges – Multicellular without – Hermaphrodites tissues – Sexual and asexual reproduction – Choanocytes (collar cells) use flagella to move water and nutrients into pores – Intracellular digestion Fig. 25.11 Animal diversity, part 2 Clicker Question Diversity 2 I. Parazoa In diploblastic animals, the inner lining of the digestive cavity or tract is derived from II. Cnidaria & Ctenophora A. Endoderm. II. Outline the B. Ectoderm. unique III. Lophotrochozoa C. Mesoderm. characteristics D. Coelom. of cnidarians and IV. Ecdysozoa ctenophores 2 Coral Box jelly Cnidaria and Ctenophora • Cnidarians – Coral; sea anemone; jellyfish; hydra; box jellies • Ctenophores – Comb jellies Sea anemone Jellyfish Hydra Comb jelly Cnidaria and Ctenophora Fig. 25.12 Coral Box jelly Cnidaria and Ctenophora • Tissues Fig. 25.12 – -

J32 the Importance of the Burgess Shale < Soft Bodied Fauna >
580 Chapter j PALEOCONTINENTS The Present is the Key to the Past: HUGH RANCE j32 The importance of the Burgess shale < soft bodied fauna > Only about 33 animal body plans are presently [sic] being used on this planet (Margulis and Schwartz, 1988). —Scott F. Gilbert, Developmental Biology, 1991.1 Almost all animal phyla known today were already present by 505 million years ago— the age of the Burgess shale, Middle Cambrian marine sediments, discovered at the Kicking Horse rim, British Columbia, in 1909 by Charles Doolittle Walcott, that provide a unique window on life without hard parts that had continued to exist shortly after the time of the Cambrian explosion (see Topic j34).2 Legend has it that Walcott, then secretary of the Smithsonian Institution, vacationing near Field, British Columbia, was thrown from a horse carrying him, when it tripped on, and split open a stray fallen slab of shale. Walcott, with his face literally rubbed in it, saw strange, but not hallucinational, forms crisply etched in black against the blue-black bedding surface of the shale: a bonanza of fossils of sea creatures without mineralized shells or backbones. Many are preserved whole; including those with articulated organic (biodegradable) exoskeletons. Details of even their soft body parts can be seen (best using PTM)3 as silvery films (formed of phyllosilicates on a coating of kerogenized carbon) that commonly outline even the most delicate structures on the fossilized animal.4 The Burgess shale is part of the Stephen Formation of greenish shales and thin-bedded limestones, which is a marine-offlap deposit between the thick, massive, carbonates of the overlying Eldon formation, and the underlying Cathedral formation.6 As referenced in the Geological Atlas of the Western Canada Sedimentary Basin - Chapter 8, the Stephen Formation has been “informally divided into a normal, ‘thin Stephen’ on the platform areas and a ‘thick Stephen’ west of the Cathedral Escarpment. -

A Paleontological Perspective of Vertebrate Origin
http://www.paper.edu.cn Chinese Science Bulletin 2003 Vol. 48 No. 8 725-735 Cover: The earliest-known and most primitive vertebrates on the A paleontological perspective Earth---Myllokunmingia fengjiaoa , (upper) Zhongjianichthys rostratus of vertebrate origin ( middle ), Haikouichthys ercaicunensis (lower left and lower right). They were SHU Degan Early Life Institute & Department of Geology, Northwest products of the early Cambrian Explosion, University, Xi’an, 710069, China; School of Earth excavated from the famous Chengjiang Sciences and Resources, China University of Geosciences, Beijing, 100083, China Lagersttat, which was formed in the (e-mail:[email protected]) eastern Yunnan about 530 millions of years ago. These ancestral vertebrates not only Abstract The Early Cambrian Haikouichthys and Haikouella have been claimed to be related to contribute in an important developed primitive separate vertebral way to our understanding of vertebrate origin, but there have elements, but also possessed principal been heated debates about how exactly they are to be interpreted. New discoveries of numerous specimens of sensory organs, including a pair of large Haikouichthys not only confirm the identity of previously lateral eyes, nostril with nasal sacs, then described structures such as the dorsal and the ventral fins, and chevron-shaped myomeres, but also reveal many new had led to the transition from acraniates to important characteristics, including sensory organs of the head craniates (true vertebrates). The (e.g. large eyes), and a prominent notochord with differentiated vertebral elements. This “first fish” appears, discoveries of these “naked” agnathans however, to retain primitive reproductive features of have pushed the earliest record of acraniates, suggesting that it is a stem-group craniates. -

Tunicata 4 Alberto Stolfi and Federico D
Tunicata 4 Alberto Stolfi and Federico D. Brown Chapter vignette artwork by Brigitte Baldrian. © Brigitte Baldrian and Andreas Wanninger. A. Stolfi Department of Biology , Center for Developmental Genetics, New York University , New York , NY , USA F. D. Brown (*) EvoDevo Laboratory, Departamento de Zoologia , Instituto de Biociências, Universidade de São Paulo , São Paulo , SP , Brazil Evolutionary Developmental Biology Laboratory, Department of Biological Sciences , Universidad de los Andes , Bogotá , Colombia Centro Nacional de Acuicultura e Investigaciones Marinas (CENAIM) , Escuela Superior Politécnica del Litoral (ESPOL) , San Pedro , Santa Elena , Ecuador e-mail: [email protected] A. Wanninger (ed.), Evolutionary Developmental Biology of Invertebrates 6: Deuterostomia 135 DOI 10.1007/978-3-7091-1856-6_4, © Springer-Verlag Wien 2015 [email protected] 136 A. Stolfi and F.D. Brown Above all , perhaps , I am indebted to a decidedly the phylogenetic relationships between the three vegetative , often beautiful , and generally obscure classes and many orders and families have yet to group of marine animals , both for their intrinsic interest and for the enjoyment I have had in search- be satisfactorily settled. Appendicularia, ing for them . N. J. Berrill (1955) Thaliacea, and Ascidiacea remain broadly used in textbooks and scientifi c literature as the three classes of tunicates; however, recent molecular INTRODUCTION phylogenies have provided support for the mono- phyly of only Appendicularia and Thaliacea, but Tunicates are a group of marine fi lter-feeding not of Ascidiacea (Swalla et al. 2000 ; animals1 that have been traditionally divided into Tsagkogeorga et al. 2009 ; Wada 1998 ). A para- three classes: (1) Appendicularia, also known as phyletic Ascidiacea calls for a reevaluation of larvaceans because their free-swimming and tunicate relationships. -

Nemertean and Phoronid Genomes Reveal Lophotrochozoan Evolution and the Origin of Bilaterian Heads
Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads Author Yi-Jyun Luo, Miyuki Kanda, Ryo Koyanagi, Kanako Hisata, Tadashi Akiyama, Hirotaka Sakamoto, Tatsuya Sakamoto, Noriyuki Satoh journal or Nature Ecology & Evolution publication title volume 2 page range 141-151 year 2017-12-04 Publisher Springer Nature Macmillan Publishers Limited Rights (C) 2017 Macmillan Publishers Limited, part of Springer Nature. Author's flag publisher URL http://id.nii.ac.jp/1394/00000281/ doi: info:doi/10.1038/s41559-017-0389-y Creative Commons Attribution 4.0 International (http://creativecommons.org/licenses/by/4.0/) ARTICLES https://doi.org/10.1038/s41559-017-0389-y Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads Yi-Jyun Luo 1,4*, Miyuki Kanda2, Ryo Koyanagi2, Kanako Hisata1, Tadashi Akiyama3, Hirotaka Sakamoto3, Tatsuya Sakamoto3 and Noriyuki Satoh 1* Nemerteans (ribbon worms) and phoronids (horseshoe worms) are closely related lophotrochozoans—a group of animals including leeches, snails and other invertebrates. Lophotrochozoans represent a superphylum that is crucial to our understand- ing of bilaterian evolution. However, given the inconsistency of molecular and morphological data for these groups, their ori- gins have been unclear. Here, we present draft genomes of the nemertean Notospermus geniculatus and the phoronid Phoronis australis, together with transcriptomes along the adult bodies. Our genome-based phylogenetic analyses place Nemertea sis- ter to the group containing Phoronida and Brachiopoda. We show that lophotrochozoans share many gene families with deu- terostomes, suggesting that these two groups retain a core bilaterian gene repertoire that ecdysozoans (for example, flies and nematodes) and platyzoans (for example, flatworms and rotifers) do not. -
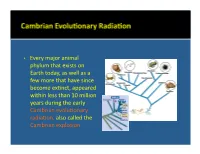
• Every Major Animal Phylum That Exists on Earth Today, As Well As A
• Every major animal phylum that exists on Earth today, as well as a few more that have since become ex:nct, appeared within less than 10 million years during the early Cambrian evolu:onary radiaon, also called the Cambrian explosion. • Phylum Pro:sta includes a diverse group of eukaryo:c microorganisms which are mostly unicellular. They are not necessarily primi:ve, although some are. Only some have a skeleton that can be preserved as a fossil. • Foraminifera and radiolaria are the most important examples. Coccolithophores, diatoms, and dinoflagellates are also examples. • Foraminifera are marine organisms that may be either planktonic, living in the water column where they float at various levels, or benthic, living on or within the seafloor sediment. • They form relavely large (0.1 mm to 5 cm) porous internal skeletons called tests through which project strands of living cytoplasm. The single- to mul:-chambered tests may be composed of organic maer, agglu:nated sand grains or sponge spicules, or calcium carbonate. • Benthic foraminifera • Planktonic foraminifera (Cambrian to Recent). (Jurassic to Recent). Oligocene Eocene • Radiolaria are planktonic marine organisms that tend to live in relavely cold water. • Their tests, oNen delicate and elaborate, are made of silica and presently accumulate on the floors of the parts of oceans that are deeper than those where foraminiferal tests are accumulang. • Radiolaria (Cambrian to • Radiolaria (Cambrian to Recent). Recent). Paleocene Radiolaria • Coccolithophores (Triassic to Recent) are extremely small marine planktonic photosynthe:c unicellular algae that are enclosed by plates of low-Mg calcite called coccoliths. Cretaceous White Cliffs of Dover, England • Diatoms (Jurassic to Recent) are small freshwater and marine planktonic photosynthe:c unicellular algae that are enclosed by a cell wall made of silica called a frustule. -

The Cambrian ''Explosion'
Perspective The Cambrian ‘‘explosion’’: Slow-fuse or megatonnage? Simon Conway Morris* Department of Earth Sciences, University of Cambridge, Cambridge CB2 3EQ, United Kingdom Clearly, the fossil record from the Cambrian period is an invaluable tool for deciphering animal evolution. Less clear, however, is how to integrate the paleontological information with molecular phylogeny and developmental biology data. Equally challenging is answering why the Cambrian period provided such a rich interval for the redeployment of genes that led to more complex bodyplans. illiam Buckland knew about it, The First Metazoans. Ediacaran assem- 1). The overall framework of early meta- WCharles Darwin characteristically blages (2, 5) are presumably integral to zoan evolution comes from molecular agonized over it, and still we do not fully understanding the roots of the Cambrian data, but they cannot provide insights into understand it. ‘‘It,’’ of course, is the seem- ‘‘explosion,’’ and this approach assumes the anatomical changes and associated ingly abrupt appearance of animals in the that the fossil record is historically valid. It changes in ecology that accompanied the Cambrian ‘‘explosion.’’ The crux of this is markedly at odds, however, with an emergence of bodyplans during the Cam- evolutionary problem can be posed as a alternative view, based on molecular data. brian explosion. The fossil record series of interrelated questions. Is it a real These posit metazoan divergences hun- provides, therefore, a unique historical event or simply an artifact of changing dreds of millions of years earlier (6, 7). As perspective. fossilization potential? If the former, how such, the origination of animals would be Only those aspects of the Ediacaran rapidly did it happen and what are its more or less coincident with the postu- record relevant to the Cambrian diversi- consequences for understanding evolu- lated ‘‘Big Bang’’ of eukaryote diversifi- fication are noted here. -

The Nervous System of the Lophophore in the Ctenostome Amathia Gracilis
Temereva and Kosevich BMC Evolutionary Biology (2016) 16:181 DOI 10.1186/s12862-016-0744-7 RESEARCH ARTICLE Open Access The nervous system of the lophophore in the ctenostome Amathia gracilis provides insight into the morphology of ancestral ectoprocts and the monophyly of the lophophorates Elena N. Temereva* and Igor A. Kosevich Abstract Background: The Bryozoa (=Ectoprocta) is a large group of bilaterians that exhibit great variability in the innervation of tentacles and in the organization of the cerebral ganglion. Investigations of bryozoans from different groups may contribute to the reconstruction of the bryozoan nervous system bauplan. A detailed investigation of the polypide nervous system of the ctenostome bryozoan Amathia gracilis is reported here. Results: The cerebral ganglion displays prominent zonality and has at least three zones: proximal, central, and distal. The proximal zone is the most developed and contains two large perikarya giving rise to the tentacle sheath nerves. The neuroepithelial organization of the cerebral ganglion is revealed. The tiny lumen of the cerebral ganglion is represented by narrow spaces between the apical projections of the perikarya of the central zone. The cerebral ganglion gives rise to five groups of main neurite bundles of the lophophore and the tentacle sheath: the circum-oral nerve ring, the lophophoral dorso-lateral nerves, the pharyngeal and visceral neurite bundles, the outer nerve ring, and the tentacle sheath nerves. Serotonin-like immunoreactive nerve system of polypide includes eight large perikarya located between tentacles bases. There are two analmost and six oralmost perikarya with prominent serotonergic “gap” between them. Based on the characteristics of their innervations, the tentacles can be subdivided into two groups: four that are near the anus and six that are near the mouth.