New Insights from Phylogenetic Analyses of Deuterostome Phyla
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"Lophophorates" Brachiopoda Echinodermata Asterozoa
Deuterostomes Bryozoa Phoronida "lophophorates" Brachiopoda Echinodermata Asterozoa Stelleroidea Asteroidea Ophiuroidea Echinozoa Holothuroidea Echinoidea Crinozoa Crinoidea Chaetognatha (arrow worms) Hemichordata (acorn worms) Chordata Urochordata (sea squirt) Cephalochordata (amphioxoius) Vertebrata PHYLUM CHAETOGNATHA (70 spp) Arrow worms Fossils from the Cambrium Carnivorous - link between small phytoplankton and larger zooplankton (1-15 cm long) Pharyngeal gill pores No notochord Peculiar origin for mesoderm (not strictly enterocoelous) Uncertain relationship with echinoderms PHYLUM HEMICHORDATA (120 spp) Acorn worms Pharyngeal gill pores No notochord (Stomochord cartilaginous and once thought homologous w/notochord) Tornaria larvae very similar to asteroidea Bipinnaria larvae CLASS ENTEROPNEUSTA (acorn worms) Marine, bottom dwellers CLASS PTEROBRANCHIA Colonial, sessile, filter feeding, tube dwellers Small (1-2 mm), "U" shaped gut, no gill slits PHYLUM CHORDATA Body segmented Axial notochord Dorsal hollow nerve chord Paired gill slits Post anal tail SUBPHYLUM UROCHORDATA Marine, sessile Body covered in a cellulose tunic ("Tunicates") Filter feeder (» 200 L/day) - perforated pharnx adapted for filtering & repiration Pharyngeal basket contractable - squirts water when exposed at low tide Hermaphrodites Tadpole larvae w/chordate characteristics (neoteny) CLASS ASCIDIACEA (sea squirt/tunicate - sessile) No excretory system Open circulatory system (can reverse blood flow) Endostyle - (homologous to thyroid of vertebrates) ciliated groove -

Evidence for Selection on a Chordate Histocompatibility Locus
ORIGINAL ARTICLE doi:10.1111/j.1558-5646.2012.01787.x EVIDENCE FOR SELECTION ON A CHORDATE HISTOCOMPATIBILITY LOCUS Marie L. Nydam,1,2,3 Alyssa A. Taylor,3 and Anthony W. De Tomaso3 1Division of Science and Mathematics, Centre College, Danville, Kentucky 40422 2E-mail: [email protected] 3Department of Molecular, Cellular, and Developmental Biology, University of California Santa Barbara, Santa Barbara, California 93106 Received June 7, 2011 Accepted July 31, 2012 Allorecognition is the ability of an organism to differentiate self or close relatives from unrelated individuals. The best known applications of allorecognition are the prevention of inbreeding in hermaphroditic species (e.g., the self-incompatibility [SI] systems in plants), the vertebrate immune response to foreign antigens mediated by MHC loci, and somatic fusion, where two genetically independent individuals physically join to become a chimera. In the few model systems where the loci governing allorecognition outcomes have been identified, the corresponding proteins have exhibited exceptional polymorphism. But information about the evolution of this polymorphism outside MHC is limited. We address this subject in the ascidian Botryllus schlosseri,where allorecognition outcomes are determined by a single locus, called FuHC (Fusion/HistoCompatibility). Molecular variation in FuHC is distributed almost entirely within populations, with very little evidence for differentiation among different populations. Mutation plays a larger role than recombination in the creation of FuHC polymorphism. A selection statistic, neutrality tests, and distribution of variation within and among different populations all provide evidence for selection acting on FuHC, but are not in agreement as to whether the selection is balancing or directional. -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -

Review Sheet for Lecture 15: Life in the Benthic Realm
Lecture 15: Life in the Benthic Realm Terminology Planktonic, nektonic, benthic, epifaunal, infaunal, sessile, mobile, bathyal, abyssal, abyssopelagic, hadopelagic, supratidal, intertidal, subtidal, spicules, spongin, radial symmetry, encruster, epidermal cell, porocyte, sclerocyte, amoebocyte, mesohyl, choanocyte, polyp, medusa, colonial, solitary, aragonite, octocoral, gorgonin, cnidocyst, nematocyst, zooxanthellae, hematypic, coral bleaching, lophophore, zoid, zooecium, zooaria, polymorphism, pedicle, helically coiled shell, radially coiled shell, exoskeleton, endoskeleton Dates: none Review Questions: Be familiar with the life and feeding modes of all of the invertebrate groups we discussed and their major morphologies, behaviors, life strategies etc. Why are so many sessile invertebrates radially symmetric? Explain the three different wall types found in sponges, what’s the point? Discuss the different roles of the different sponge cells How do hematypic corals contribute to the creation of different nearshore environments and conditions? Describe the community created by an innkeeper worm How does the skeleton of a brachiopod differ from that of a clam? How do the shells of clams, snails, and nautiloids differ? Classification: Phylum Porifera Class Demospongiae (soft sponges) Class Calcarea (calcareous sponges) Class Hexactinellida (glass sponges) Phylum Cnidaria Class Anthozoa -stony corals -sea fans -soft corals -sea anemones -sea pens Phylum Annelida (worms) -bristleworms -innkeeper worm -lugworms -spaghetti worm -feather duster -catworm Phylum Bryozoa (moss-animals) Phylum Mollusca -Class Bivalvia (clams) -Class Gastropoda (snails and slugs) -Class Cephalopoda (squid, octopus, nautiloid) -Class Scaphopoda (tusk shells) -Class Polyplacophora (chitons) . -

Development of the Annelid Axochord: Insights Into Notochord Evolution Antonella Lauri Et Al
RESEARCH | REPORTS ORIGIN OF NOTOCHORD by double WMISH (Fig. 2, F to L). Although none of the genes were exclusively expressed in the annelid mesodermal midline, their combined Development of the annelid coexpression was unique to these cells (implying that mesodermal midline in annelids and chor- damesoderm in vertebrates are more similar to axochord: Insights into each other than to any other tissue). It is unlikely that the molecular similarity between annelid notochord evolution and vertebrate mesodermal midline is due to in- dependent co-option of a conserved gene cas- Antonella Lauri,1*† Thibaut Brunet,1* Mette Handberg-Thorsager,1,2‡ sette, because this would require either that this Antje H.L. Fischer,1§ Oleg Simakov,1 Patrick R. H. Steinmetz,1‖ Raju Tomer,1,2¶ cassette was active elsewhere in the body (which Philipp J. Keller,2 Detlev Arendt1,3# is not the case) or that multiple identical inde- pendent events of co-option occurred (which is The origin of chordates has been debated for more than a century, with one key issue being unparsimonious). As in vertebrates, the meso- the emergence of the notochord. In vertebrates, the notochord develops by convergence dermal midline resembles the neuroectodermal and extension of the chordamesoderm, a population of midline cells of unique molecular midline, which expresses foxD, foxA, netrin, slit, identity. We identify a population of mesodermal cells in a developing invertebrate, the marine and noggin (figs. S6 and S7) but not brachyury or annelid Platynereis dumerilii, that converges and extends toward the midline and expresses a twist. However, unlike in chicken (10), the an- notochord-specific combination of genes. -

Collin Et Al. Page 1 of 31 Unexpected Molecular and Morphological
Unexpected Molecular and Morphological Diversity of Hemichordate Larvae from the Neotropics Rachel Collin1*, Dagoberto E. Venera-Pontón1, Amy C. Driskell2, Kenneth S. Macdonald III2, Michael J. Boyle3 1Smithsonian Tropical Research Institute, Apartado Postal 0843-03092, Balboa Ancon, Panama. 2Laboratories of Analytical Biology, National Museum of Natural History, Smithsonian Institution, Washington DC, USA 3Smithsonian Marine Station, Fort Pierce, Florida *Corresponding author: e-mail: [email protected]; (202) 633-4700 x28766. Address for correspondence: STRI, Unit 9100, Box 0948, DPO AA 34002, USA. Invertebrate Biology Keywords: Tropical East Pacific, Panama, Caribbean, meroplankton, enteropneust, invertebrate, larval morphology Running Head: Neotropic Hemichordate Larval Diversity September 2019 Collin et al. Page 1 of 31 Abstract The diversity of tropical marine invertebrates is poorly documented, especially those groups for which collecting adults is difficult. We collected the planktonic tornaria larvae of hemichordates (acorn worms) to assess their hidden diversity in the Neotropics. Larvae were retrieved in plankton tows from waters of the Pacific and Caribbean coasts of Panama, followed by DNA barcoding of mitochondrial cytochrome c oxidase subunit I (COI) and 16S ribosomal DNA to estimate their diversity in the region. With moderate sampling efforts, we discovered 6 operational taxonomic units (OTUs) in the Bay of Panama on the Pacific coast, in contrast to the single species previously recorded for the entire Tropical Eastern Pacific. We found 8 OTUs in Bocas del Toro Province on the Caribbean coast, compared to 7 species documented from adults in the entire Caribbean. All OTUs differed from each other and from named acorn worm sequences in GenBank by >10% pairwise distance in COI and >2% in 16S. -

Mitochondrial Genomes of Two Polydora
www.nature.com/scientificreports OPEN Mitochondrial genomes of two Polydora (Spionidae) species provide further evidence that mitochondrial architecture in the Sedentaria (Annelida) is not conserved Lingtong Ye1*, Tuo Yao1, Jie Lu1, Jingzhe Jiang1 & Changming Bai2 Contrary to the early evidence, which indicated that the mitochondrial architecture in one of the two major annelida clades, Sedentaria, is relatively conserved, a handful of relatively recent studies found evidence that some species exhibit elevated rates of mitochondrial architecture evolution. We sequenced complete mitogenomes belonging to two congeneric shell-boring Spionidae species that cause considerable economic losses in the commercial marine mollusk aquaculture: Polydora brevipalpa and Polydora websteri. The two mitogenomes exhibited very similar architecture. In comparison to other sedentarians, they exhibited some standard features, including all genes encoded on the same strand, uncommon but not unique duplicated trnM gene, as well as a number of unique features. Their comparatively large size (17,673 bp) can be attributed to four non-coding regions larger than 500 bp. We identifed an unusually large (putative) overlap of 14 bases between nad2 and cox1 genes in both species. Importantly, the two species exhibited completely rearranged gene orders in comparison to all other available mitogenomes. Along with Serpulidae and Sabellidae, Polydora is the third identifed sedentarian lineage that exhibits disproportionally elevated rates of mitogenomic architecture rearrangements. Selection analyses indicate that these three lineages also exhibited relaxed purifying selection pressures. Abbreviations NCR Non-coding region PCG Protein-coding gene Metazoan mitochondrial genomes (mitogenomes) usually encode the set of 37 genes, comprising 2 rRNAs, 22 tRNAs, and 13 proteins, encoded on both genomic strands. -

Animal Phylum Poster Porifera
Phylum PORIFERA CNIDARIA PLATYHELMINTHES ANNELIDA MOLLUSCA ECHINODERMATA ARTHROPODA CHORDATA Hexactinellida -- glass (siliceous) Anthozoa -- corals and sea Turbellaria -- free-living or symbiotic Polychaetes -- segmented Gastopods -- snails and slugs Asteroidea -- starfish Trilobitomorpha -- tribolites (extinct) Urochordata -- tunicates Groups sponges anemones flatworms (Dugusia) bristleworms Bivalves -- clams, scallops, mussels Echinoidea -- sea urchins, sand Chelicerata Cephalochordata -- lancelets (organisms studied in detail in Demospongia -- spongin or Hydrazoa -- hydras, some corals Trematoda -- flukes (parasitic) Oligochaetes -- earthworms (Lumbricus) Cephalopods -- squid, octopus, dollars Arachnida -- spiders, scorpions Mixini -- hagfish siliceous sponges Xiphosura -- horseshoe crabs Bio1AL are underlined) Cubozoa -- box jellyfish, sea wasps Cestoda -- tapeworms (parasitic) Hirudinea -- leeches nautilus Holothuroidea -- sea cucumbers Petromyzontida -- lamprey Mandibulata Calcarea -- calcareous sponges Scyphozoa -- jellyfish, sea nettles Monogenea -- parasitic flatworms Polyplacophora -- chitons Ophiuroidea -- brittle stars Chondrichtyes -- sharks, skates Crustacea -- crustaceans (shrimp, crayfish Scleropongiae -- coralline or Crinoidea -- sea lily, feather stars Actinipterygia -- ray-finned fish tropical reef sponges Hexapoda -- insects (cockroach, fruit fly) Sarcopterygia -- lobed-finned fish Myriapoda Amphibia (frog, newt) Chilopoda -- centipedes Diplopoda -- millipedes Reptilia (snake, turtle) Aves (chicken, hummingbird) Mammalia -

Invertebrates Invertebrates: • Are Animals Without Backbones • Represent 95% of the Animal Kingdom Animal Diversity Morphological Vs
Invertebrates Invertebrates: • Are animals without backbones • Represent 95% of the animal kingdom Animal Diversity Morphological vs. Molecular Character Phylogeny? A tree is a hypothesis supported or not supported by evidence. Groupings change as new evidence become available. Sponges - Porifera Natural Bath Sponges – over-collected, now uncommon Sponges • Perhaps oldest animal phylum (Ctenphora possibly older) • may represent several old phyla, some now extinct ----------------Ctenophora? Sponges - Porifera • Mostly marine • Sessile animals • Lack true tissues; • Have only a few cell types, cells kind of independent • Most have no symmetry • Body resembles a sac perforated with holes, system of canals. • Strengthened by fibers of spongin, spicules Sponges have a variety of shapes Sponges Pores Choanocyte Amoebocyte (feeding cell) Skeletal Water fiber flow Central cavity Flagella Choanocyte in contact with an amoebocyte Sponges - Porifera • Sessile filter feeder • No mouth • Sac-like body, perforated by pores. • Interior lined by flagellated cells (choanocytes). Flagellated collar cells generate a current, draw water through the walls of the sponge where food is collected. • Amoeboid cells move around in the mesophyll and distribute food. Sponges - Porifera Grantia x.s. Sponge Reproduction Asexual reproduction • Fragmentation or by budding. • Sponges are capable of regeneration, growth of a whole from a small part. Sexual reproduction • Hermaphrodites, produce both eggs and sperm • Eggs and sperm released into the central cavity • Produces -

Zootaxa, a Taxonomic Revision of the Family Harrimaniidae (Hemichordata
Zootaxa 2408: 1–30 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) A taxonomic revision of the family Harrimaniidae (Hemichordata: Enteropneusta) with descriptions of seven species from the Eastern Pacific C. DELAND1, C. B. CAMERON1,6, K. P. RAO2,5, W. E. RITTER3,5 & T. H. BULLOCK4,5 1Sciences biologiques, Université de Montréal, C.P. 6128, Succ. Centre-ville, Montreal, QC, H3C 3J7, Canada 2Department of Zoology, Bangalore University, Bangalore, India 3Scripps Institution of Oceanography, University of California San Diego, La Jolla, California, U.S.A. 4Scripps Institution of Oceanography and Department of Neurosciences, School of Medicine, University of California San Diego, La Jolla, California, U.S.A. 5Deceased 6Corresponding author. E-mail: [email protected] Abstract The family Harrimaniidae (Hemichordata: Enteropneusta) is revised on the basis of morphological characters. The number of harrimaniid genera is increased to nine by the addition of Horstia n. gen., Mesoglossus n. gen., Ritteria n. gen. and Saxipendium, a genus previously assigned to the monospecific family Saxipendiidae. The number of species is increased to 34, resulting from the description of five new species from the eastern Pacific — Horstia kincaidi, Mesoglossus intermedius, M. macginitiei, Protoglossus mackiei and Ritteria ambigua. A description is supplied for a sixth harrimaniid species, Stereobalanus willeyi Ritter & Davis, 1904, which previously had the status of a nomen nudum. Four harrimaniids previously assigned to the genus Saccoglossus are transfered to the genus Mesoglossus — M. bournei, M. caraibicus, M. gurneyi and M. pygmaeus, while Saccoglossus borealis is reassigned to the genus Harrimania. -
S I Section 4
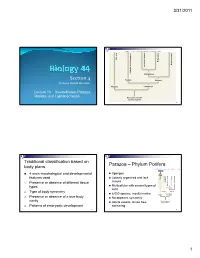
3/31/2011 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Porifera Ecdysozoa Deuterostomia Lophotrochozoa Cnidaria and Ctenophora Cnidaria and Protostomia SSiection 4 Radiata Bilateria Professor Donald McFarlane Parazoa Eumetazoa Lecture 13 Invertebrates: Parazoa, Radiata, and Lophotrochozoa Ancestral colonial choanoflagellate 2 Traditional classification based on Parazoa – Phylum Porifera body plans Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Parazoa 4 main morphological and developmental Sponges features used Loosely organized and lack Porifera Ecdysozoa Cnidaria and Ctenophora tissues euterostomia photrochozoa D 1. o Presence or absence of different tissue L Multicellular with several types of types Protostomia cells 2. Type of body symmetry Radiata Bilateria 8,000 species, mostly marine Parazoa Eumetazoa 3. Presence or absence of a true body No apparent symmetry cavity Ancestral colonial Adults sessile, larvae free- choanoflagellate 4. Patterns of embryonic development swimming 3 4 1 3/31/2011 Water drawn through pores (ostia) into spongocoel Flows out through osculum Reproduce Choanocytes line spongocoel Sexually Most hermapppgggphrodites producing eggs and sperm Trap and eat small particles and plankton Gametes are derived from amoebocytes or Mesohyl between choanocytes and choanocytes epithelial cells Asexually Amoebocytes absorb food from choanocytes, Small fragment or bud may detach and form a new digest it, and carry -

Hemichordate Phylogeny: a Molecular, and Genomic Approach By
Hemichordate Phylogeny: A molecular, and genomic approach by Johanna Taylor Cannon A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama May 4, 2014 Keywords: phylogeny, evolution, Hemichordata, bioinformatics, invertebrates Copyright 2014 by Johanna Taylor Cannon Approved by Kenneth M. Halanych, Chair, Professor of Biological Sciences Jason Bond, Associate Professor of Biological Sciences Leslie Goertzen, Associate Professor of Biological Sciences Scott Santos, Associate Professor of Biological Sciences Abstract The phylogenetic relationships within Hemichordata are significant for understanding the evolution of the deuterostomes. Hemichordates possess several important morphological structures in common with chordates, and they have been fixtures in hypotheses on chordate origins for over 100 years. However, current evidence points to a sister relationship between echinoderms and hemichordates, indicating that these chordate-like features were likely present in the last common ancestor of these groups. Therefore, Hemichordata should be highly informative for studying deuterostome character evolution. Despite their importance for understanding the evolution of chordate-like morphological and developmental features, relationships within hemichordates have been poorly studied. At present, Hemichordata is divided into two classes, the solitary, free-living enteropneust worms, and the colonial, tube- dwelling Pterobranchia. The objective of this dissertation is to elucidate the evolutionary relationships of Hemichordata using multiple datasets. Chapter 1 provides an introduction to Hemichordata and outlines the objectives for the dissertation research. Chapter 2 presents a molecular phylogeny of hemichordates based on nuclear ribosomal 18S rDNA and two mitochondrial genes. In this chapter, we suggest that deep-sea family Saxipendiidae is nested within Harrimaniidae, and Torquaratoridae is affiliated with Ptychoderidae.