Nozzle Selection and Adjuvant Impact on the Efficacy of Glyphosate And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Growth Regulation and Other Secondary Effects of Herbicides Edivaldo D
Weed Science 2010 58:351–354 Growth Regulation and Other Secondary Effects of Herbicides Edivaldo D. Velini, Maria L. B. Trindade, Luis Rodrigo M. Barberis, and Stephen O. Duke* As all herbicides act on pathways or processes crucial to plants, in an inhibitory or stimulatory way, low doses of any herbicide might be used to beneficially modulate plant growth, development, or composition. Glyphosate, the most used herbicide in the world, is widely applied at low rates to ripen sugarcane. Low rates of glyphosate also can stimulate plant growth (this effect is called hormesis). When applied at recommended rates for weed control, glyphosate can inhibit rust diseases in glyphosate-resistant wheat and soybean. Fluridone blocks carotenoid biosynthesis by inhibition of phytoene desaturase and is effective in reducing the production of abscisic acid in drought-stressed plants. Among the acetolactate synthase inhibitors, sulfometuron-methyl is widely used to ripen sugarcane and imidazolinones can be used to suppress turf species growth. The application of protoporphyrinogen oxidase inhibitors can trigger plant defenses against pathogens. Glufosinate, a glutamine synthetase inhibitor, is also known to improve the control of plant diseases. Auxin agonists (i.e., dicamba and 2,4-D) are effective, low-cost plant growth regulators. Currently, auxin agonists are still used in tissue cultures to induce somatic embryogenesis and to control fruit ripening, to reduce drop of fruits, to enlarge fruit size, or to extend the harvest period in citrus orchards. At low doses, triazine herbicides stimulate growth through beneficial effects on nitrogen metabolism and through auxin-like effects. Thus, sublethal doses of several herbicides have applications other than weed control. -

PPO2 Mutations in Amaranthus Palmeri:Implications on Cross-Resistance
agriculture Article PPO2 Mutations in Amaranthus palmeri: Implications on Cross-Resistance Pâmela Carvalho-Moore 1,2 , Gulab Rangani 1, James Heiser 3, Douglas Findley 4, Steven J. Bowe 4 and Nilda Roma-Burgos 1,* 1 Department of Crop, Soil and Environmental Sciences, University of Arkansas, Fayetteville, AR 72704, USA; [email protected] (P.C.-M.); [email protected] (G.R.) 2 Former Cell and Molecular Biology Program, University of Arkansas, Fayetteville, AR 72704, USA 3 Fisher Delta Research Center, College of Agriculture, University of Missouri, Portageville, MO 63873, USA; [email protected] 4 BASF Corporation, Research Triangle Park, NC 27709, USA; douglas.fi[email protected] (D.F.); [email protected] (S.J.B.) * Correspondence: [email protected] Abstract: In Arkansas, resistance to protoporphyrinogen IX oxidase (PPO)-inhibiting herbicides in Amaranthus palmeri S. Wats. is mainly due to target site mutations. Although A. palmeri PPO-mutations are well investigated, the cross-resistance that each ppo mutant endows to weed populations is not yet well understood. We aimed to evaluate the response of PPO-resistant A. palmeri accessions, harboring the ppo2 mutations DG210 and G399A, to multiple PPO-inhibiting herbicides. Six resistant and one susceptible field accessions were subjected to a dose–response assay with fomesafen, and selected survivors from different fomesafen doses were genotyped to characterize the mutation profile. The level of resistance to fomesafen was determined and a cross-resistance assay was conducted with 1 Citation: Carvalho-Moore, P.; and 2 times the labeled doses of selected PPO herbicides. The accession with higher predicted dose Rangani, G.; Heiser, J.; Findley, D.; to control 50% of the population (ED50) had a higher frequency of DG210-homozygous survivors. -

Herbicide Mode of Action Table High Resistance Risk
Herbicide Mode of Action Table High resistance risk Chemical family Active constituent (first registered trade name) GROUP 1 Inhibition of acetyl co-enzyme A carboxylase (ACC’ase inhibitors) clodinafop (Topik®), cyhalofop (Agixa®*, Barnstorm®), diclofop (Cheetah® Gold* Decision®*, Hoegrass®), Aryloxyphenoxy- fenoxaprop (Cheetah®, Gold*, Wildcat®), fluazifop propionates (FOPs) (Fusilade®), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) Cyclohexanediones (DIMs) butroxydim (Factor®*), clethodim (Select®), profoxydim (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) Phenylpyrazoles (DENs) pinoxaden (Axial®) GROUP 2 Inhibition of acetolactate synthase (ALS inhibitors), acetohydroxyacid synthase (AHAS) Imidazolinones (IMIs) imazamox (Intervix®*, Raptor®), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, Lightning®*, Midas®* OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) Pyrimidinyl–thio- bispyribac (Nominee®), pyrithiobac (Staple®) benzoates Sulfonylureas (SUs) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*, Trimac Plus®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron (Logran®, Logran® B-Power®*), tribenuron (Express®), -

Weed Control in Direct-Seeded Field Pea Gregory J
Weed Control in Direct-seeded Field Pea Gregory J. Endres and Blaine G. Schatz Weed control and field pea response to selected soil- and POST-applied herbicides were evaluated in a randomized complete-block design with three replicates. The experiment was conducted on a Heimdahl loam soil with 6.7 pH and 2.9% organic matter at the NDSU Carrington Research Extension Center. Herbicide treatments were applied to 5- by 25-ft plots with a pressurized hand-held plot sprayer at 17 gal/A and 30 psi through 8002 flat-fan nozzles. Fall sulfentrazone treatments were applied October 25, 2004 to a moist soil surface with 47 F, 71% RH, 15% clear sky, and 11 mph wind. On April 28, 2005, inoculated 'Integra' field pea was seeded into standing wheat stubble in 7-inch rows at a rate of 300,000 pure live seeds/A. PRE treatments were applied to a dry soil surface on April 30 with 31 F, 64% RH, 30% clear sky, and 10 mph wind. Rainfall totaled 1.22 inches 8 d following PRE application. The trial area was treated on May 6 with a PRE burn-down application of glyphosate at 0.75 lb ae/A plus ammonium sulfate at 1% v/v. The early POST (EPOST) treatment was applied on May 23 with 73 F, 35% RH, 100% cloudy sky, and 6 mph wind to 2-inch tall field pea, 1- to 2-leaf green and yellow foxtail, 0.5-inch tall common lambsquarters, 0.5-inch tall prostrate and redroot pigweed, and 0.5-inch tall wild buckwheat. -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Target-Site Mutations Conferring Herbicide Resistance
plants Review Target-Site Mutations Conferring Herbicide Resistance Brent P. Murphy and Patrick J. Tranel * Department of Crop Sciences, University of Illinois, Urbana, IL 61801, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-217-333-1531 Received: 4 September 2019; Accepted: 26 September 2019; Published: 28 September 2019 Abstract: Mutations conferring evolved herbicide resistance in weeds are known in nine different herbicide sites of action. This review summarizes recently reported resistance-conferring mutations for each of these nine target sites. One emerging trend is an increase in reports of multiple mutations, including multiple amino acid changes at the glyphosate target site, as well as mutations involving two nucleotide changes at a single amino acid codon. Standard reference sequences are suggested for target sites for which standards do not already exist. We also discuss experimental approaches for investigating cross-resistance patterns and for investigating fitness costs of specific target-site mutations. Keywords: D1 protein; acetolactate synthase; tubulin; ACCase; EPSPS; phytoene desaturase; PPO; glutamine synthetase; auxin 1. Introduction Herbicide-resistance mechanisms broadly fall under two categories: target-site mechanisms and non-target-site mechanisms [1,2]. The former involves a change to the molecular target of the herbicide (usually an enzyme) that decreases its affinity for the herbicide. Although much less common, target-site resistance can also occur via increased expression of the target, which results in more herbicide required to achieve a lethal effect [3,4]. Non-target-site resistance encompasses any mechanism that reduces the amount of herbicide that reaches the target site, or that ameliorates the effect of the herbicide despite its inhibition of the target site. -

RR Program's RCL Spreadsheet Update
RR Program’s RCL Spreadsheet Update March 2017 RR Program RCL Spreadsheet Update DNR-RR-052e The Wisconsin DNR Remediation and Redevelopment Program (RR) has updated the numerical soil standards in the August 2015 DNR-RR- 052b RR spreadsheet of residual contaminant levels (RCLs). The RCLs were determined using the U.S. EPA RSL web- calculator by accepting EPA exposure defaults, with the exception of using Chicago, IL, for the climatic zone. This documentThe U.S. provides EPA updateda summary its Regionalof changes Screening to the direct-contact Level (RSL) RCLs website (DC-RCLs) in June that2015. are To now reflect in the that March 2017 spreadsheet.update, the The Wisconsin last page ofDNR this updated document the has numerical the EPA exposuresoil standards, parameter or residual values usedcontaminant in the RCL levels calculations. (RCLs), in the Remediation and Redevelopment program’s spreadsheet of RCLs. This document The providesU.S. EPA a RSL summary web-calculator of the updates has been incorporated recently updated in the Julyso that 2015 the spreadsheet.most up-to-date There toxicity were values no changes for chemi - cals madewere certainlyto the groundwater used in the RCLs,RCL calculations. but there are However, many changes it is important in the industrial to note that and the non-industrial web-calculator direct is only a subpartcontact of the (DC) full RCLsEPA RSL worksheets. webpage, Tables and that 1 andthe other 2 of thissubparts document that will summarize have important the DC-RCL explanatory changes text, generic tablesfrom and the references previous have spreadsheet yet to be (Januaryupdated. -

US EPA, Pesticide Product Label, A335.06,09/21/2020
[Note to reviewer: [Text] in brackets denotes optional or explanatory language [Note to reviewer: {Text} in braces denotes where in the final label text will appear {BOOKLET FRONT PANEL LANGUAGE} S-METOLACHLOR GROUP 15 HERBICIDE METRIBUZIN GROUP 5 HERBICIDE FOMESAFEN GROUP 14 HERBICIDE A335.06[™] [Alternate Brand Name: Statler] [Herbicide for preemergent control of certain grasses and broadleaf weeds in Soybeans] ACTIVE INGREDIENTS: (% by weight) S-Metolachlor*………………………………………….……………………………………………………………………………..…………………… 36.29% Metribuzin**………………………………………….……………………………………………………………………………..………………………. 8.05% Fomesafen***………………………………………….……………………………………………………………………………..……………………... 7.16% OTHER INGREDIENTS: ……………………………………………………..…………………………………………………………………………….. 48.5% TOTAL …………………………………………………………………………………………………………….……………………………………………. 100.0% *contains 3.39 Ib of S-metolachlor per gallon **contains 0.75 Ib of metribuzin per gallon ***contains 0.67 Ib of fomesafen acid per gallon KEEP OUT OF REACH OF CHILDREN CAUTION Si usted no entiende la etiqueta, busque a alguien para que se la explique a usted en detalle. (If you do not understand the label, find someone to explain it to you in detail.) See [below] [inside label booklet] for [additional] [First Aid,] [Precautionary Statements] and [Directions for Use]. EPA Reg. No.: 91234-201 EPA Est. No.: Net Weight: Manufactured for: Atticus, LLC 5000 CentreGreen Way, Suite 100 Cary, NC 27513 1 {LANGUAGE INSIDE BOOKLET} FIRST AID If on skin or Take off contaminated clothing. clothing: Rinse skin immediately with plenty of water for 15-20 minutes. Call a poison control center or doctor for treatment advice. If swallowed: Call a poison control center or doctor immediately for treatment advice. Have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by the poison control center or doctor. -

US EPA, Pesticide Product Label, Metolachlor + Metribuzin EC,04/15
U.S. ENVIRONMENTAL PROTECTION AGENCY EPA Reg. Number: Date of Issuance: Office of Pesticide Programs Registration Division (7505P) 42750-360 4/15/20 1200 Pennsylvania Ave., N.W. Washington, D.C. 20460 NOTICE OF PESTICIDE: Term of Issuance: X Registration Reregistration Conditional (under FIFRA, as amended) Name of Pesticide Product: METOLACHLOR + METRIBUZIN EC Name and Address of Registrant (include ZIP Code): Albaugh, LLC P.O. Box 2127 Valdosta, GA 31604-2127 Note: Changes in labeling differing in substance from that accepted in connection with this registration must be submitted to and accepted by the Registration Division prior to use of the label in commerce. In any correspondence on this product always refer to the above EPA registration number. On the basis of information furnished by the registrant, the above named pesticide is hereby registered under the Federal Insecticide, Fungicide and Rodenticide Act. Registration is in no way to be construed as an endorsement or recommendation of this product by the Agency. In order to protect health and the environment, the Administrator, on his motion, may at any time suspend or cancel the registration of a pesticide in accordance with the Act. The acceptance of any name in connection with the registration of a product under this Act is not to be construed as giving the registrant a right to exclusive use of the name or to its use if it has been covered by others. This product is conditionally registered in accordance with FIFRA section 3(c)(7)(A). You must comply with the following conditions: 1. Submit and/or cite all data required for registration/reregistration/registration review of your product under FIFRA when the Agency requires all registrants of similar products to submit such data. -

AP-42, CH 9.2.2: Pesticide Application
9.2.2PesticideApplication 9.2.2.1General1-2 Pesticidesaresubstancesormixturesusedtocontrolplantandanimallifeforthepurposesof increasingandimprovingagriculturalproduction,protectingpublichealthfrompest-bornediseaseand discomfort,reducingpropertydamagecausedbypests,andimprovingtheaestheticqualityofoutdoor orindoorsurroundings.Pesticidesareusedwidelyinagriculture,byhomeowners,byindustry,andby governmentagencies.Thelargestusageofchemicalswithpesticidalactivity,byweightof"active ingredient"(AI),isinagriculture.Agriculturalpesticidesareusedforcost-effectivecontrolofweeds, insects,mites,fungi,nematodes,andotherthreatstotheyield,quality,orsafetyoffood.Theannual U.S.usageofpesticideAIs(i.e.,insecticides,herbicides,andfungicides)isover800millionpounds. AiremissionsfrompesticideusearisebecauseofthevolatilenatureofmanyAIs,solvents, andotheradditivesusedinformulations,andofthedustynatureofsomeformulations.Mostmodern pesticidesareorganiccompounds.EmissionscanresultdirectlyduringapplicationorastheAIor solventvolatilizesovertimefromsoilandvegetation.Thisdiscussionwillfocusonemissionfactors forvolatilization.Thereareinsufficientdataavailableonparticulateemissionstopermitemission factordevelopment. 9.2.2.2ProcessDescription3-6 ApplicationMethods- Pesticideapplicationmethodsvaryaccordingtothetargetpestandtothecroporothervalue tobeprotected.Insomecases,thepesticideisapplieddirectlytothepest,andinotherstothehost plant.Instillothers,itisusedonthesoilorinanenclosedairspace.Pesticidemanufacturershave developedvariousformulationsofAIstomeetboththepestcontrolneedsandthepreferred -

CLARC Excerpt
Washington State Department of Ecology - CLARC Air Table (Methods B and C) - February 2021 February 2021 S S CPFi S CPFo S Air Air RfC o RfDi o Inhalation RfDo o Oral o Air Air Method C Method C Inhalation u Inhalation IUR u Cancer Oral u Cancer u Method B Method B Noncancer Cancer Reference Reference Inhalation Potency Reference Potency Noncancer Cancer (Eq. 750-1 (Eq. 750-2 Chemical Data Links to r r r r Concentration c Dose Unit Risk c Factor Dose c Factor c (Eq. 750-1) (Eq. 750-2) adjusted) adjusted) 3 3 -1 CAS No. Group Chemical Name Important Notes (mg/m ) e (mg/kg-day) (µg/m ) e (kg-day/mg) (mg/kg-day) e (kg-day/mg) e (µg/m³) (µg/m³) (µg/m³) (µg/m³) 83-32-9 PAHs acenaphthene 6.00E-02 I 30560-19-1 Pesticides acephate 1.20E-03 O 75-07-0 VOCs acetaldehyde 9.00E-03 I 2.57E-03 2.20E-06 I 7.70E-03 4.10E+00 1.10E+00 9.00E+00 1.10E+01 34256-82-1 Pesticides acetochlor 2.00E-02 I 67-64-1 VOCs acetone 3.10E+01 A 8.86E+00 9.00E-01 I 1.40E+04 3.10E+04 75-86-5 VOCs acetone cyanohydrin 2.00E-03 X 5.71E-04 9.10E-01 2.00E+00 75-05-8 VOCs acetonitrile 6.00E-02 I 1.71E-02 2.70E+01 6.00E+01 98-86-2 SVOCs acetophenone 1.00E-01 I 62476-59-9 Herbicides acifluorfen, sodium 1.30E-02 I 107-02-8 VOCs acrolein 2.00E-05 I 5.71E-06 5.00E-04 I 9.10E-03 2.00E-02 79-06-1 VOCs acrylamide 6.00E-03 I 1.71E-03 1.00E-04 I-M 3.50E-01 2.00E-03 I 5.00E-01 I-M 2.70E+00 6.60E-03 6.00E+00 2.50E-01 79-10-7 VOCs acrylic acid 1.00E-03 I 2.86E-04 5.00E-01 I 4.60E-01 1.00E+00 107-13-1 VOCs acrylonitrile 2.00E-03 I 5.71E-04 6.80E-05 I 2.38E-01 4.00E-02 A 5.40E-01 I 9.10E-01 3.70E-02 -
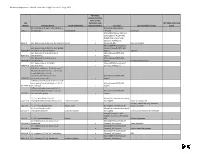
Chemicals of High Concern List (Sorted Alphabetically), July 2010
Minnesota Department of Health, Chemicals of High Concern list, July 1, 2010 Persistent, Bioaccumulative, Toxic or very CAS Persistent, very HPV (2006 and 3 of 4 Number Chemical Name Health endpoint(s) Bioaccumulative Source(s) Use example(s) or class years) (S)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2- Maine (EU Reproductive 5543-57-7 benzopyrone Reproduction Toxicant) Sunscreen Maine (CA Prop 65; IARC; EU Carcinogen; NTP 11th ROC; OSPAR Chemicals of High Concern); WA Appen1; 91-94-1 [1,1'-biphenyl]-4,4'-diamine, 3,3'-dichloro-Cancer x Minnesota HRL Dye, curing agent Maine (OSPAR Chemicals of [1,1'-biphenyl]-4,4'-diamine, N,N'-bis(2,4- Concern; Canada PBiT); WA 29398-96-7 dinitrophenyl)-3,3'-dimethoxy- x Appen1 Colorant [1,1'-Biphenyl]-4-ol, 3,4',5-tris(1,1- Maine (Canada PBiT); WA 6257-39-2 dimethylethyl)- x Appen1 [1,1'-Biphenyl]-4-ol, 3,4'-bis(1,1- Maine (Canada PBiT); WA 42479-88-9 dimethylethyl)- x Appen1 Chemical intermediate [1,1'-biphenyl]-4-ol, 3,5-bis(1,1- Maine (OSPAR Chemicals of 2668-47-5 dimethylethyl)- x Concern); WA Appen1 [2,6'-Bibenzothiazole]-7-sulfonic acid, 2'- (4-aminophenyl)-6-methyl-, diazotized, coupled with diazotized 4- aminobenzenesulfonic acid and Maine (Canada PBiT); WA 91696-90-1 resorcinol, sodium salts x Appen1 1(2H)-Quinolineethanol, 6-[(2-chloro-4,6- dinitrophenyl) azo]-3,4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63133-84-6 tetramethyl- x Appen1 1(2H)-Quinolinepropanamide, 6-(2,2- dicyanoethenyl)-3, 4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63467-15-2 tetramethyl-N-phenyl- x Appen1 1,1,1,2-Tetrachloro-2,2-bis(4-