Electrochemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cell Notation Practical Galvanic Cells -Batteries
Basic Redox Vocabulary • Write reactions for each of the following: • oxidation of metallic nickel by BiO+ • reduction of Zn2+ by hydroxide ion • reaction of Fe 2+ with Hg2+ 2+ • reaction of Cd with NO2 • AgI acting as an oxidizing agent toward Sn 2+ • What’s wrong with • The oxidation of Cr by Cl- • The reduction of Co 2+ by Ag+ Cell Notation • As was noted earlier, galvanic cells normally consist of two distinct regions, one housing the oxidation half and the other the reduction half. There is a simplified notation form that allows one to represent the cell easily( text p 798- 799). • The oxidation is written on the left and the reduction on the right. starting with the anode material and ending with the cathode material. • phase boundaries represented with single vertical lines “ |” • the physical separation between the two half cells is a double v ertical line “||” if it’s a salt bridge and with a single broken vertical line, “!”, if it’s a liquid junction • within each have cell, the species are written in a reactant-product order, separated by commas if they are in the same phase. Acid/base components should be included • The electrode material may be actively participating in the redox chemistry (active electrode) or merely providing surface for the electron transfer (passive or inert electrode, usually graphite or Pt) • Represent the following as galvanic cells(assume the reactions are spontaneous as written) • Tl(s) + Cd 2+ ó Tl + + Cd(s) - 2+ 2+ • Pb(s) + MnO4 ó Pb + Mn (acid) 2+ 4+ • O2(g) + Sn ó H2O + Sn Practical Galvanic Cells -batteries • Batteries represent the most common application of the electrochemical cell. -

Environmental Protection Agency
Friday, December 19, 2003 Part II Environmental Protection Agency 40 CFR Part 63 National Emission Standards for Hazardous Air Pollutants: Mercury Emissions from Mercury Cell Chlor-Alkali Plants; Final Rule VerDate jul<14>2003 15:14 Dec 18, 2003 Jkt 203001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\19DER2.SGM 19DER2 70904 Federal Register / Vol. 68, No. 244 / Friday, December 19, 2003 / Rules and Regulations ENVIRONMENTAL PROTECTION types of sources (usually in the Information or other information whose AGENCY elemental or inorganic forms) transports disclosure is restricted by statute. through the atmosphere and eventually The official public docket is the 40 CFR Part 63 deposits onto land or water bodies. collection of materials that is available [OAR–2002–0017; FRL–7551–5] When mercury is deposited to surface for public viewing. The EPA Docket waters, natural processes (bacterial) can RIN 2060–AE85 Center Public Reading Room is open transform some of the mercury into from 8:30 a.m. to 4:30 p.m., Monday methylmercury that accumulates in fish. through Friday, excluding legal National Emission Standards for Ingestion is the primary exposure route Hazardous Air Pollutants: Mercury holidays. The telephone number for the of interest for methylmercury. The Reading Room is (202) 566–1744, and Emissions From Mercury Cell Chlor- health effect of greatest concern due to Alkali Plants the telephone number for the Air Docket methylmercury is neurotoxicity, is (202) 566–1742. AGENCY: Environmental Protection particularly with respect to fetuses and Agency (EPA). young children. Electronic Docket Access. You may access the final rule electronically ACTION: Final rule. -

1. the Diagram Below Shows an Electrolytic Cell Using Graphite Electrodes
1 Quiz (More Complicated Questions) 1. The diagram below shows an electrolytic cell using graphite electrodes. (a) Explain why the bulb does not light up when distilled water is used as electrolyte. (b) Explain why the bulb lights up when hydrogen chloride gas is passed into distilled water. (c) During electrolysis, colourless gas bubbles are produced at both electrodes A and B. What is the gas produced at each electrode? (d) Write half equations for the reactions taking place at electrodes A and B respectively. (e) Explain why the rates of formation of gas at the two electrodes are different. 2. The following set-up shows the electrolysis of dilute copper(II) sulphate solution with different electrodes in two electrolytic cells, X and Y, connected in series. 2 (a) (i) Identify the anode and the cathode in electrolytic cell X. (ii) Write the half equation for the reaction taking place at each electrode in electrolytic cell X. (iii) State the expected observable change(s) at each electrode in electrolytic cell X. (b) (i) Identify the anode and the cathode in electrolytic cell Y. (ii) Write the half equation for the reaction taking place at each electrode in electrolytic cell Y. (iii) State the expected observable change(s) at each electrode in electrolytic cell Y. (c) Explain why different products are produced at electrodes A and C. (d) State and explain the change of copper(II) sulphate solution in each electrolytic cell after electrolysis. 3. A microscale experiment is carried out to study the electrolysis of very dilute sodium chloride solution containing some universal indicator. -

Voltaic Cells
Voltaic Cells Tro Chapter 19 – Electrochemistry 19.3 Voltaic(or Galvanic) Cells: Generating Electricity from Spontaneous Chemical Reactions Electric Current Flowing Directly Between Atoms Tro, Chemistry: A Molecular Approach 2 Electrochemical Cells Voltaic (Galvanic) ΔG < 0 to Electrolytic Δ G > 0 uses electrical generate electrical energy. energy to drive non-spontaneous process. Electrochemical Cells • Oxidation and reduction half-reactions are kept separate in half-cells. • Electron flow through a wire along with ion flow through a solution constitutes an electric circuit. • It requires a conductive solid electrode to allow the transfer of electrons. – Through external circuit – Metal or graphite • Requires ion exchange between the Daniell Cell two half-cells of the system. – Electrolyte Definitions Anode Salt Bridge • Electrode where oxidation always occurs • An inverted, U-shaped tube containing a • More negatively charged electrode in strong electrolyte and connecting the two voltaic cell half-cells. • Typically made of metal that is oxidized Cathode • Electrode where reduction always occurs Potential Difference • More positively charged electrode in • The difference in potential energy voltaic cell between the reactants and products. • Typically metal that is produced by reduction (Caused by an electric field resulting from the charge difference on the two If the redox reaction involves the oxidation or electrodes.) reduction of an ion to a different oxidation state, or the oxidation or reduction of a gas, we Cell Potential (Ecell or emf) may use an inert electrode. • The potential difference between the anode and the cathode in a voltaic cell. • An inert electrode is one that not does participate in the reaction but just provides a surface on which the transfer of electrons can take place. -
HCL Software's
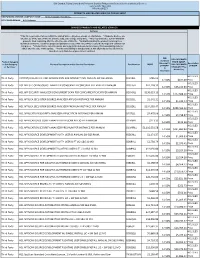
IBM Branded, Fujistu Branded and Panasonic Branded Products and Related Services and Cloud Services Contract DIR-TSO-3999 PRICING SHEET PRODUCTS AND RELATED SERVICES PRICING SHEET RESPONDING VENDOR COMPANY NAME:____Sirius Computer Solutions_______________ PROPOSED BRAND:__HCL Software__________________________ BRANDED PRODUCTS AND RELATED SERVICES Software * This file is generated for use within the United States. All prices shown are US Dollars. * Products & prices are effective as of the date of this file and are subject to change at any time. * HCL may announce new or withdraw products from marketing after the effective date of this file. * Nothwithstanding the product list and prices identified on this file, customer proposals/quotations issued will reflect HCL's current offerings and commercial list prices. * Product list is not all inclusive and may not include products removed from availability (sale) or added after the date of this update. * Product availability is not guaranteed. Not all products listed below are found on every State/Local government contract. DIR DIR CUSTOMER Customer Product Category PRICE (MSRP- Discount % Description or SubCategory Product Description and/or Service Description Part Number MSRP DIR CUSTOMER off MSRP* of MSRP or Services* DISCOUNT Plus (2 Admin Fee) Decimals) HCL SLED Third Party CNTENT/COLLAB ACC AND WEBSPH PRTL SVR INTRANET PVU ANNUAL SW S&S RNWL E045KLL $786.02 14.50% $677.09 Price HCL SLED Third Party HCL APP SEC OPEN SOURCE ANALYZER CONSCAN PER CONCURRENT EVENT PER ANNUM D20H6LL $41,118.32 -

Monitoring Enterprise Collaboration Platform Change and the Building of Digital Transformation Capabilities
Monitoring Enterprise Collaboration Platform Change and the Building of Digital Transformation Capabilities: An Information Infrastructure Perspective by Clara Sabine Nitschke Approved Dissertation thesis for the partial fulfilment of the requirements for a Doctor of Economics and Social Sciences (Dr. rer. pol.) Fachbereich 4: Informatik Universität Koblenz-Landau Chair of PhD Board: Prof. Dr. Ralf Lämmel Chair of PhD Commission: Prof. Dr. Viorica Sofronie-Stokkermans Examiner and Supervisor: Prof. Dr. Susan P. Williams Further Examiners: Prof. Dr. Petra Schubert, Prof. Dr. Catherine A. Hardy Date of the doctoral viva: 28/07/2021 Acknowledgements Many thanks to all the people who supported me on my PhD journey, a life-changing experience. This work was funded by two research grants from the Deutsche Forschungsgemeinschaft (DFG). The related projects were designed as a joint work between two research groups at the University Koblenz-Landau. I am especially grateful to my supervisor Prof. Dr. Sue Williams who provided invaluable support throughout my whole project, particularly through her expertise, as well as her research impulses and discussions. Still, she gave me the freedom to shape my research, so many thanks! Further, I would like to thank my co-advisor Prof. Dr. Petra Schubert for very constructive feedback through the years. I am thankful for having had the unique opportunity to be part of the Center for Enterprise Information Research (CEIR) team with excellent researchers and the IndustryConnect initiative, which helped me bridge the gap between academia and ‘real world’ cases. IndustryConnect, founded by Prof. Dr. Schubert and Prof. Dr. Williams, enabled me to participate in long-term practice-oriented research with industry, a privilege that most other doctoral students cannot enjoy. -

Investor Release
INVESTOR RELEASE Noida, India, January 15th, 2021 Revenue at US$ 10,022 mn; up 3.6% YoY in US$ and Constant Currency EBITDA margin at 26.5%, (US GAAP); EBITDA margin at 27.4% (Ind AS); EBIT margin at 21.5% Net Income at US$ 1781 mn (Net Income margin at 17.8%) up 19.8% YoY Revenue at ` 74,327 crores; up 9.2% YoY Net Income at ` 13,202 crores; up 26.0% YoY Revenue at US$ 2,617 mn; up 4.4% QoQ & up 2.9% YoY Revenue in Constant Currency; up 3.5% QoQ & up 1.1% YoY EBITDA margin at 28.2%, (US GAAP); EBITDA margin at 29.1% (Ind AS); EBIT margin at 22.9% Net Income at US$ 540 mn (Net Income margin at 20.6%) up 27.3% QoQ & up 26.5% YoY Revenue at ` 19,302 crores; up 3.8% QoQ & up 6.4% YoY Net Income at ` 3,982 crores; up 26.7% QoQ & up 31.1% YoY Revenue expected to grow QoQ between 2% to 3% in constant currency for Q4, FY’21, including DWS contribution. EBIT expected to be between 21.0% and 21.5% for FY’21 Financial Highlights 2 Corporate Overview 4 Performance Trends 5 Financials in US$ 18 Cash and Cash Equivalents, Investments & Borrowings 21 Revenue Analysis at Company Level 22 Constant Currency Reporting 23 Client Metrics 24 Headcount 24 Financials in ` 25 - 1 - (Amount in US $ Million) CALENDAR YEAR QUARTER ENDED PARTICULARS CY’20 Margin YoY 31-Dec-2020 Margin QoQ YoY Revenue 10,022 3.6% 2,617 4.4% 2.9% Revenue Growth 3.6% 3.5% 1.1% (Constant Currency) EBITDA 2,655 26.5% 19.9% 738 28.2% 10.5% 17.7% EBIT 2,155 21.5% 16.5% 599 22.9% 10.6% 16.4% Net Income 1,781 17.8% 19.8% 540 20.6% 27.3% 26.5% (Amount in ` Crores) CALENDAR YEAR QUARTER ENDED -

Galvanic Cell Notation • Half-Cell Notation • Types of Electrodes • Cell
Galvanic Cell Notation ¾Inactive (inert) electrodes – not involved in the electrode half-reaction (inert solid conductors; • Half-cell notation serve as a contact between the – Different phases are separated by vertical lines solution and the external el. circuit) 3+ 2+ – Species in the same phase are separated by Example: Pt electrode in Fe /Fe soln. commas Fe3+ + e- → Fe2+ (as reduction) • Types of electrodes Notation: Fe3+, Fe2+Pt(s) ¾Active electrodes – involved in the electrode ¾Electrodes involving metals and their half-reaction (most metal electrodes) slightly soluble salts Example: Zn2+/Zn metal electrode Example: Ag/AgCl electrode Zn(s) → Zn2+ + 2e- (as oxidation) AgCl(s) + e- → Ag(s) + Cl- (as reduction) Notation: Zn(s)Zn2+ Notation: Cl-AgCl(s)Ag(s) ¾Electrodes involving gases – a gas is bubbled Example: A combination of the Zn(s)Zn2+ and over an inert electrode Fe3+, Fe2+Pt(s) half-cells leads to: Example: H2 gas over Pt electrode + - H2(g) → 2H + 2e (as oxidation) + Notation: Pt(s)H2(g)H • Cell notation – The anode half-cell is written on the left of the cathode half-cell Zn(s) → Zn2+ + 2e- (anode, oxidation) + – The electrodes appear on the far left (anode) and Fe3+ + e- → Fe2+ (×2) (cathode, reduction) far right (cathode) of the notation Zn(s) + 2Fe3+ → Zn2+ + 2Fe2+ – Salt bridges are represented by double vertical lines ⇒ Zn(s)Zn2+ || Fe3+, Fe2+Pt(s) 1 + Example: A combination of the Pt(s)H2(g)H Example: Write the cell reaction and the cell and Cl-AgCl(s)Ag(s) half-cells leads to: notation for a cell consisting of a graphite cathode - 2+ Note: The immersed in an acidic solution of MnO4 and Mn 4+ reactants in the and a graphite anode immersed in a solution of Sn 2+ overall reaction are and Sn . -

Federal Register/Vol. 67, No. 128
Federal Register / Vol. 67, No. 128 / Wednesday, July 3, 2002 / Proposed Rules 44713 TABLE 9 TO SUBPART IIIII OF PART 63.—APPLICABILITY OF GENERAL PROVISIONS TO SUBPART IIIII—Continued Applies to Subpart Citation Subject IIIII Explanation § 63.10(d)(3) ........................................... Reporting Opacity or VE Observations No ......................... Subpart IIIII does not have opacity and visible emission standards. § 63.11 .................................................... Flares ................................................... No ......................... Subpart IIIII does not require flares. § 63.12 .................................................... Delegation ............................................ Yes. § 63.13 .................................................... Addresses ............................................ Yes. § 63.14 .................................................... Incorporation by Reference .................. Yes. § 63.15 .................................................... Availability of Information ..................... Yes. [FR Doc. 02–15873 Filed 7–2–02; 8:45 am] A–2002–09, U.S. EPA, 401 M Street, Standards Division, U.S. EPA, Research BILLING CODE 6560–50–P SW., Washington, DC 20460. Triangle Park, NC 27711. The EPA will Public Hearing. If a public hearing is disclose information identified as CBI held, it will be held at the new EPA only to the extent allowed by the ENVIRONMENTAL PROTECTION facility complex in Research Triangle procedures set forth in 40 CFR part 2. AGENCY Park, North -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -

Lithium Batteries
Batteries General planning of the „Lithium Batteries” lab for the European Master 2007/8 Warsaw University of Technology, Departament of Inorganic Chemistry and Solid State Technology, Dr Marek Marcinek Objectives: Students will: follow the development of primary and secondary lithium batteries become familiar with different types of batteries explore the applications of batteries study the major components of lithium (ion) cells learn which batteries can be recycled realize the economic and environmental advantages of using rechargeable batteries Needs: Room with avialiables 2 desks and 2 computers or Multimedia Projector Potentiostat/galvanostat with galvanic cycle mode. Suplementary materials needed for: Building the simple battery (included Volta cell) Daniell cell Leclanche Cell Commercially available variety of Li-bat (also disassembled in a controlled mode) BDS software Safety issues: Safety rules Read directions carefully before you begin any experiments. Clear an area to work. Wash your hands thoroughly after experimenting Do always wear eye protection Keep all chemicals away from your eyes and mouth Do not eat and drink in your experiment area Put all pieces of equipment away when finished using them General First aid information Indicate the person who should IMMIDIATELLY inform the teacher and ask the other person to help you out if needed. Eyes: rinse immediately with water. Remove contact lenses if wearing any. Flush eyes with water for 15 min Swallowed: Rinse mouth Drink glass full of water or milk. DO NOT INDUCE VOMITING SKIN: Flush skin thoroughly with water. In all cases, get immediate medical attention if an emergency exists. Bring the chemical container with you. 1 Materials given to students/preparation: Exemplary 5 scientific papers (or any material found related to the Battery Performance, Design, Safety, Application) for individual preparation. -

PLUMBING DICTIONARY Sixth Edition
as to produce smooth threads. 2. An oil or oily preparation used as a cutting fluid espe cially a water-soluble oil (such as a mineral oil containing- a fatty oil) Cut Grooving (cut groov-ing) the process of machining away material, providing a groove into a pipe to allow for a mechani cal coupling to be installed.This process was invented by Victau - lic Corp. in 1925. Cut Grooving is designed for stanard weight- ceives or heavier wall thickness pipe. tetrafluoroethylene (tet-ra-- theseveral lower variouslyterminal, whichshaped re or decalescensecryolite (de-ca-les-cen- ming and flood consisting(cry-o-lite) of sodium-alumi earthfluo-ro-eth-yl-ene) by alternately dam a colorless, thegrooved vapors tools. from 4. anonpressure tool used by se) a decrease in temperaturea mineral nonflammable gas used in mak- metalworkers to shape material thatnum occurs fluoride. while Usedheating for soldermet- ing a stream. See STANK. or the pressure sterilizers, and - spannering heat resistantwrench and(span-ner acid re - conductsto a desired the form vapors. 5. a tooldirectly used al ingthrough copper a rangeand inalloys which when a mixed with phosphoric acid.- wrench)sistant plastics 1. one ofsuch various as teflon. tools to setthe theouter teeth air. of Sometimesaatmosphere circular or exhaust vent. See change in a structure occurs. Also used for soldering alumi forAbbr. tightening, T.F.E. or loosening,chiefly Brit.: orcalled band vapor, saw. steam,6. a tool used to degree of hazard (de-gree stench trap (stench trap) num bronze when mixed with nutsthermal and bolts.expansion 2. (water) straightenLOCAL VENT.