The Pennsylvania State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 1 DNA Barcodes Reveal Deeply Neglected Diversity and Numerous
Page 1 of 57 1 DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in 2 Madagascar 3 4 5 Carlos Lopez-Vaamonde1,2, Lucas Sire2, Bruno Rasmussen2, Rodolphe Rougerie3, 6 Christian Wieser4, Allaoui Ahamadi Allaoui 5, Joël Minet3, Jeremy R. deWaard6, Thibaud 7 Decaëns7, David C. Lees8 8 9 1 INRA, UR633, Zoologie Forestière, F- 45075 Orléans, France. 10 2 Institut de Recherche sur la Biologie de l’Insecte, UMR 7261 CNRS Université de Tours, UFR 11 Sciences et Techniques, Tours, France. 12 3Institut de Systématique Evolution Biodiversité (ISYEB), Muséum national d'Histoire naturelle, 13 CNRS, Sorbonne Université, EPHE, 57 rue Cuvier, CP 50, 75005 Paris, France. 14 4 Landesmuseum für Kärnten, Abteilung Zoologie, Museumgasse 2, 9020 Klagenfurt, Austria 15 5 Department of Entomology, University of Antananarivo, Antananarivo 101, Madagascar 16 6 Centre for Biodiversity Genomics, University of Guelph, 50 Stone Road E., Guelph, ON 17 N1G2W1, Canada 18 7Centre d'Ecologie Fonctionnelle et Evolutive (CEFE UMR 5175, CNRS–Université de Genome Downloaded from www.nrcresearchpress.com by UNIV GUELPH on 10/03/18 19 Montpellier–Université Paul-Valéry Montpellier–EPHE), 1919 Route de Mende, F-34293 20 Montpellier, France. 21 8Department of Life Sciences, Natural History Museum, Cromwell Road, SW7 5BD, UK. 22 23 24 Email for correspondence: [email protected] For personal use only. This Just-IN manuscript is the accepted prior to copy editing and page composition. It may differ from final official version of record. 1 Page 2 of 57 25 26 Abstract 27 Madagascar is a prime evolutionary hotspot globally, but its unique biodiversity is under threat, 28 essentially from anthropogenic disturbance. -

DNA Barcodes Reveal Deeply Neglected Diversity and Numerous Invasions of Micromoths in Madagascar
Genome DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in Madagascar Journal: Genome Manuscript ID gen-2018-0065.R2 Manuscript Type: Article Date Submitted by the 17-Jul-2018 Author: Complete List of Authors: Lopez-Vaamonde, Carlos; Institut National de la Recherche Agronomique (INRA), ; Institut de Recherche sur la Biologie de l’Insecte (IRBI), Sire, Lucas; Institut de Recherche sur la Biologie de l’Insecte Rasmussen,Draft Bruno; Institut de Recherche sur la Biologie de l’Insecte Rougerie, Rodolphe; Institut Systématique, Evolution, Biodiversité (ISYEB), Wieser, Christian; Landesmuseum für Kärnten Ahamadi, Allaoui; University of Antananarivo, Department Entomology Minet, Joël; Institut de Systematique Evolution Biodiversite deWaard, Jeremy; Biodiversity Institute of Ontario, University of Guelph, Decaëns, Thibaud; Centre d'Ecologie Fonctionnelle et Evolutive (CEFE UMR 5175, CNRS–Université de Montpellier–Université Paul-Valéry Montpellier–EPHE), , CEFE UMR 5175 CNRS Lees, David; Natural History Museum London Keyword: Africa, invasive alien species, Lepidoptera, Malaise trap, plant pests Is the invited manuscript for consideration in a Special 7th International Barcode of Life Issue? : https://mc06.manuscriptcentral.com/genome-pubs Page 1 of 57 Genome 1 DNA barcodes reveal deeply neglected diversity and numerous invasions of micromoths in 2 Madagascar 3 4 5 Carlos Lopez-Vaamonde1,2, Lucas Sire2, Bruno Rasmussen2, Rodolphe Rougerie3, 6 Christian Wieser4, Allaoui Ahamadi Allaoui 5, Joël Minet3, Jeremy R. deWaard6, Thibaud 7 Decaëns7, David C. Lees8 8 9 1 INRA, UR633, Zoologie Forestière, F- 45075 Orléans, France. 10 2 Institut de Recherche sur la Biologie de l’Insecte, UMR 7261 CNRS Université de Tours, UFR 11 Sciences et Techniques, Tours, France. -

The Genomes of Polyextremophilic Cyanidiales Contain 1% 2 Horizontally Transferred Genes with Diverse Adaptive Functions 3 4 Alessandro W
bioRxiv preprint doi: https://doi.org/10.1101/526111; this version posted January 23, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 The genomes of polyextremophilic Cyanidiales contain 1% 2 horizontally transferred genes with diverse adaptive functions 3 4 Alessandro W. Rossoni1#, Dana C. Price2, Mark Seger3, Dagmar Lyska1, Peter Lammers3, 5 Debashish Bhattacharya4 & Andreas P.M. Weber1* 6 7 1Institute of Plant Biochemistry, Cluster of Excellence on Plant Sciences (CEPLAS), Heinrich 8 Heine University, Universitätsstraße 1, 40225 Düsseldorf, Germany 9 2Department of Plant Biology, Rutgers University, New Brunswick, NJ 08901, USA 10 3Arizona Center for Algae Technology and Innovation, Arizona State University, Mesa, AZ 11 85212, USA 12 4Department of Biochemistry and Microbiology, Rutgers University, New Brunswick, NJ 13 08901, USA 14 15 *Corresponding author: Prof. Dr. Andreas P.M. Weber, 16 e-mail: [email protected] 17 bioRxiv preprint doi: https://doi.org/10.1101/526111; this version posted January 23, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 18 Abstract 19 The role and extent of horizontal gene transfer (HGT) in eukaryotes are hotly disputed topics 20 that impact our understanding regarding the origin of metabolic processes and the role of 21 organelles in cellular evolution. -

Floral Volatiles from Vigna Unguiculata Are Olfactory and Gustatory Stimulants for Oviposition by the Bean Pod Borer Moth Maruca Vitrata
Article Floral Volatiles from Vigna unguiculata Are Olfactory and Gustatory Stimulants for Oviposition by the Bean Pod Borer Moth Maruca vitrata Bo Feng 1, Kai Qian 1 and Yong-Jun Du 1,2,* 1 Institute of Health and Environmental Ecology, Wenzhou Medical University, University Town, Wenzhou 325035, Zhejiang, China; [email protected] (B.F.); [email protected] (K.Q.) 2 Institute of Pesticide and Environmental Toxicology, Zhejiang University, 866 Yuhangtang Rd., Hangzhou 310058, Zhejiang, China * Correspondence: [email protected]; Tel.: +86-571-88982517 Academic Editor: Brian T. Forschler Received: 8 March 2017; Accepted: 6 June 2017; Published: 9 June 2017 Abstract: We investigated the role of floral odors from cowpea, Vigna unguiculata (L.), in mediating oviposition of the bean pod borer moth, Maruca vitrata, a serious pest of grain legumes that flies to host plants at the flowering stage and oviposits onto flowers and buds. The flower of the host plant V. unguiculata was a stimulus for egg-laying by M. vitrata in an oviposition bioassay. Commercial longifolene, β-caryophyllene, linalool, geraniol, and (Z)-3-hexenyl acetate were used as stimulus. Each one elicited dose-dependent electroantennogram responses in female M. vitrata, and all but longifolene stimulated oviposition, when presented singly. Beta-caryophyllene was the most active stimulant, similar to that of the flower of V. unguiculata, and eliciting a dose-dependent oviposition response. Either olfaction or gustation was sufficient to mediate an oviposition response to V. unguiculata floral volatiles: intact M. vitrata responded to β-caryophyllene whether or not they could contact the source of the volatiles, and females with amputated antennae responded if allowed to contact the source. -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -
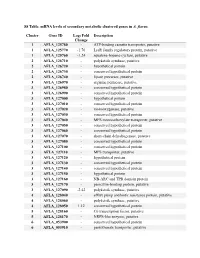
S8 Table. Mrna Levels of Secondary Metabolic Clustered Genes in A
S8 Table. mRNA levels of secondary metabolic clustered genes in A. flavus. Cluster Gene ID Log2 Fold Description Change 1 AFLA_125780 - ATP-binding cassette transporter, putative 1 AFLA_125770 -1.76 LysR family regulatory protein, putative 1 AFLA_125760 -1.24 squalene-hopene-cyclase, putative 2 AFLA_126710 - polyketide synthase, putative 2 AFLA_126720 - hypothetical protein 2 AFLA_126730 - conserved hypothetical protein 2 AFLA_126740 - lipase precursor, putative 3 AFLA_126970 - arginine permease, putative 3 AFLA_126980 - conserved hypothetical protein 3 AFLA_126990 - conserved hypothetical protein 3 AFLA_127000 - hypothetical protein 3 AFLA_127010 - conserved hypothetical protein 3 AFLA_127020 - monooxygenase, putative 3 AFLA_127030 - conserved hypothetical protein 3 AFLA_127040 - MFS monocarboxylate transporter, putative 3 AFLA_127050 - conserved hypothetical protein 3 AFLA_127060 - conserved hypothetical protein 3 AFLA_127070 - short-chain dehydrogenase, putative 3 AFLA_127080 - conserved hypothetical protein 3 AFLA_127100 - conserved hypothetical protein 3 AFLA_127110 - MFS transporter, putative 3 AFLA_127120 - hypothetical protein 3 AFLA_127130 - conserved hypothetical protein 3 AFLA_127140 - conserved hypothetical protein 3 AFLA_127150 - hypothetical protein 3 AFLA_127160 - NB-ARC and TPR domain protein 3 AFLA_127170 - penicillin-binding protein, putative 3 AFLA_127090 -2.42 polyketide synthase, putative 4 AFLA_128040 - efflux pump antibiotic resistance protein, putative 4 AFLA_128060 - polyketide synthase, putative 4 AFLA_128050 -

Comparative Genomics of Bradyrhizobium Japonicum CPAC
Siqueira et al. BMC Genomics 2014, 15:420 http://www.biomedcentral.com/1471-2164/15/420 RESEARCH ARTICLE Open Access Comparative genomics of Bradyrhizobium japonicum CPAC 15 and Bradyrhizobium diazoefficiens CPAC 7: elite model strains for understanding symbiotic performance with soybean Arthur Fernandes Siqueira1,2†, Ernesto Ormeño-Orrillo3†,RangelCelsoSouza4,ElisetePainsRodrigues5, Luiz Gonzaga Paula Almeida4, Fernando Gomes Barcellos5, Jesiane Stefânia Silva Batista6, Andre Shigueyoshi Nakatani2, Esperanza Martínez-Romero3, Ana Tereza Ribeiro Vasconcelos4 and Mariangela Hungria1,2* Abstract Background: The soybean-Bradyrhizobium symbiosis can be highly efficient in fixing nitrogen, but few genomic sequences of elite inoculant strains are available. Here we contribute with information on the genomes of two commercial strains that are broadly applied to soybean crops in the tropics. B. japonicum CPAC 15 (=SEMIA 5079) is outstanding in its saprophytic capacity and competitiveness, whereas B. diazoefficiens CPAC 7 (=SEMIA 5080) is known for its high efficiency in fixing nitrogen. Both are well adapted to tropical soils. The genomes of CPAC 15 and CPAC 7 were compared to each other and also to those of B. japonicum USDA 6T and B. diazoefficiens USDA 110T. Results: Differences in genome size were found between species, with B. japonicum having larger genomes than B. diazoefficiens. Although most of the four genomes were syntenic, genome rearrangements within and between species were observed, including events in the symbiosis island. In addition to the symbiotic region, several genomic islands were identified. Altogether, these features must confer high genomic plasticity that might explain adaptation and differences in symbiotic performance. It was not possible to attribute known functions to half of the predicted genes. -

University of Copenhagen, Copenhagen, Denmark
The multiple strategies of an insect herbivore to overcome plant cyanogenic glucoside defence Pentzold, Stefan; Zagrobelny, Mika; Roelsgaard, Pernille Sølvhøj; Møller, Birger Lindberg; Bak, Søren Published in: PLOS ONE DOI: 10.1371/journal.pone.0091337 Publication date: 2014 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Pentzold, S., Zagrobelny, M., Roelsgaard, P. S., Møller, B. L., & Bak, S. (2014). The multiple strategies of an insect herbivore to overcome plant cyanogenic glucoside defence. PLOS ONE, 9(3), [e91337]. https://doi.org/10.1371/journal.pone.0091337 Download date: 23. sep.. 2021 The Multiple Strategies of an Insect Herbivore to Overcome Plant Cyanogenic Glucoside Defence Stefan Pentzold, Mika Zagrobelny, Pernille Sølvhøj Roelsgaard, Birger Lindberg Møller, Søren Bak* Plant Biochemistry Laboratory and Villum research center ‘Plant Plasticity’, Department of Plant and Environmental Sciences, University of Copenhagen, Copenhagen, Denmark Abstract Cyanogenic glucosides (CNglcs) are widespread plant defence compounds that release toxic hydrogen cyanide by plant b- glucosidase activity after tissue damage. Specialised insect herbivores have evolved counter strategies and some sequester CNglcs, but the underlying mechanisms to keep CNglcs intact during feeding and digestion are unknown. We show that CNglc-sequestering Zygaena filipendulae larvae combine behavioural, morphological, physiological and biochemical strategies at different time points during feeding and digestion to avoid toxic hydrolysis of the CNglcs present in their Lotus food plant, i.e. cyanogenesis. We found that a high feeding rate limits the time for plant b-glucosidases to hydrolyse CNglcs. Larvae performed leaf-snipping, a minimal disruptive feeding mode that prevents mixing of plant b-glucosidases and CNglcs. -

Purification and Characterization of a Novel Nitrilase of Rhodococcus
JOURNAL OF BACTERIOLOGY, Sept. 1990, p. 4807-4815 Vol. 172, No. 9 0021-9193/90/094807-09$02.00/0 Copyright X3 1990, American Society for Microbiology Purification and Characterization of a Novel Nitrilase of Rhodococcus rhodochrous K22 That Acts on Aliphatic Nitriles MICHIHIKO KOBAYASHI,* NORIYUKI YANAKA, TORU NAGASAWA, AND HIDEAKI YAMADA Department ofAgricultural Chemistry, Faculty ofAgriculture, Kyoto University, Sakyo-ku, Kyoto 606, Japan Received 18 April 1990/Accepted 8 June 1990 A novel nitrilase that preferentially catalyzes the hydrolysis of aliphatic nitriles to the corresponding carboxylic acids and ammonia was found in the cells of a facultative crotononitrile-utilizing actinomycete isolated from soil. The strain was taxonomically studied and identified as Rhodococcus rhodochrous. The nitrilase was purified, with 9.08% overall recovery, through five steps from a cell extract of the stain. After the last step, the purified enzyme appeared to be homogeneous, as judged by polyacrylamide gel electrophoresis, analytical centrifugation, and double immunodiffusion in agarose. The relative molecular weight values for the native enzyme, estimated from the ultracentrifugal equilibrium and by high-performance liquid chromatog- raphy, were approximately 604,000 + 30,000 and 650,000, respectively, and the enzyme consisted of 15 to 16 subunits identical in molecular weight (41,000). The enzyme acted on aliphatic olefinic nitriles such as crotononitrile and acrylonitrile as the most suitable substrates. The apparent Km values for crotononitrile and acrylonitrile were 18.9 and 1.14 mM, respectively. The nitrilase also catalyzed the direct hydrolysis of saturated aliphatic nitriles, such as valeronitrile, 4-chlorobutyronitrile, and glutaronitrile, to the correspond- ing acids without the formation of amide intermediates. -

Redalyc.New and Interesting Portuguese Lepidoptera Records from 2007 (Insecta: Lepidoptera)
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Corley, M. F. V.; Marabuto, E.; Maravalhas, E.; Pires, P.; Cardoso, J. P. New and interesting Portuguese Lepidoptera records from 2007 (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 36, núm. 143, septiembre, 2008, pp. 283-300 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45512164002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 283-300 New and interesting Po 4/9/08 17:37 Página 283 SHILAP Revta. lepid., 36 (143), septiembre 2008: 283-300 CODEN: SRLPEF ISSN:0300-5267 New and interesting Portuguese Lepidoptera records from 2007 (Insecta: Lepidoptera) M. F. V. Corley, E. Marabuto, E. Maravalhas, P. Pires & J. P. Cardoso Abstract 38 species are added to the Portuguese Lepidoptera fauna and two species deleted, mainly as a result of fieldwork undertaken by the authors in the last year. In addition, second and third records for the country and new food-plant data for a number of species are included. A summary of papers published in 2007 affecting the Portuguese fauna is included. KEY WORDS: Insecta, Lepidoptera, geographical distribution, Portugal. Novos e interessantes registos portugueses de Lepidoptera em 2007 (Insecta: Lepidoptera) Resumo Como resultado do trabalho de campo desenvolvido pelos autores principalmente no ano de 2007, são adicionadas 38 espécies de Lepidoptera para a fauna de Portugal e duas são retiradas. -

Weather and Pests
Volume 52 Number 02 April 20, 2007 Weather and Pests The last remnants of the winter season have passed and a series of annual spring events has begun to unfold. Temperatures have finally moderated, reaching the 50s and 60s during the daytime, and farm activity has progressed in the past few days. Relatively little planting has been done but many fields are prepared. Insect activity has also escalated noticeably. The first round of early-season orchard pests, which includes spotted tentiform leafminer and redbanded leafroller moths, has taken flight throughout the south. In addition, the first overwintered alfalfa weevils were swept from fields earlier in the week and a few more black cutworm moths made their way into the state. Growing Degree Days through 04/19/07 were GDD 50F 2006 Sine 48F 40F Historical GDD Dubuque, IA 138 148 134 321 March 1-April 19 Lone Rock 125 144 118 302 Beloit 130 160 126 312 Madison 107 130 102 274 Sullivan 108 142 101 268 Juneau 102 129 97 261 Waukesha 110 123 102 270 Hartford 104 123 98 260 Racine 103 104 99 259 Milwaukee 100 107 94 251 Appleton 84 109 78 221 Green Bay 73 84 67 204 Big Flats 102 138 94 253 Hancock 96 132 89 241 Port Edwards 98 136 89 235 La Crosse 128 162 124 308 Eau Claire 99 149 91 243 Cumberland 81 123 70 203 Bayfield 40 71 33 140 Predicted mortality Wausau 75 113 68 200 Medford 71 115 65 192 of overwintered bean leaf beetles Crivitz 51 84 45 169 Crandon 55 98 44 160 WEB: http://pestbulletin.wi.gov z EMAIL: [email protected] z VOLUME 52 Issue No. -

2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory
2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory ........................................................................................... 3 Alaska ... ........................................ ............................................................... 3 LEPIDOPTERISTS Zone 2: British Columbia .................................................... ........................ ............ 6 Idaho .. ... ....................................... ................................................................ 6 Oregon ........ ... .... ........................ .. .. ............................................................ 10 SOCIETY Volume 53 Supplement Sl Washington ................................................................................................ 14 Zone 3: Arizona ............................................................ .................................... ...... 19 The Lepidopterists' Society is a non-profo California ............... ................................................. .............. .. ................... 2 2 educational and scientific organization. The Nevada ..................................................................... ................................ 28 object of the Society, which was formed in Zone 4: Colorado ................................ ... ............... ... ...... ......................................... 2 9 May 1947 and formally constituted in De Montana .................................................................................................... 51 cember