Scientific Evidence for Pre-Columbian Transoceanic Voyages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University of Southampton Research Repository Eprints Soton
University of Southampton Research Repository ePrints Soton Copyright © and Moral Rights for this thesis are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given e.g. AUTHOR (year of submission) "Full thesis title", University of Southampton, name of the University School or Department, PhD Thesis, pagination http://eprints.soton.ac.uk UNIVERSITY OF SOUTHAMPTON FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES SCHOOL OF OCEAN AND EARTH SCIENCE RELATIONSHIP BETWEEN WOOD DENSITY AND ULTRASOUND PROPAGATION VELOCITY: A NON-DESTRUCTIVE EVALUATION OF WATERLOGGED ARCHAEOLOGICAL WOOD STATE OF PRESERVATION BASED ON ITS UNDERWATER ACOUSTIC PROPERTIES Angeliki Zisi Thesis for the degree of Doctor of Philosophy October 2015 UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES SCHOOL OF OCEAN AND EARTH SCIENCE Thesis for the degree of Doctor of Philosophy RELATIONSHIP BETWEEN WOOD DENSITY AND ULTRASOUND PROPAGATION VELOCITY: A NON-DESTRUCTIVE EVALUATION OF WATERLOGGED ARCHAEOLOGICAL WOOD STATE OF PRESERVATION BASED ON ITS UNDERWATER ACOUSTIC PROPERTIES Angeliki Zisi With current progress in marine geophysics equipment, survey and processing techniques, we can be now closer to support needs emerging after decades of maritime archaeology and conservation practice worldwide. -

Spring 2014 Commencement Program
TE TA UN S E ST TH AT I F E V A O O E L F A DITAT DEUS N A E R R S I O Z T S O A N Z E I A R I T G R Y A 1912 1885 ARIZONA STATE UNIVERSITY COMMENCEMENT AND CONVOCATION PROGRAM Spring 2014 May 12 - 16, 2014 THE NATIONAL ANTHEM THE STAR SPANGLED BANNER O say can you see, by the dawn’s early light, What so proudly we hailed at the twilight’s last gleaming? Whose broad stripes and bright stars through the perilous fight O’er the ramparts we watched, were so gallantly streaming? And the rockets’ red glare, the bombs bursting in air Gave proof through the night that our flag was still there. O say does that Star-Spangled Banner yet wave O’er the land of the free and the home of the brave? ALMA MATER ARIZONA STATE UNIVERSITY Where the bold saguaros Raise their arms on high, Praying strength for brave tomorrows From the western sky; Where eternal mountains Kneel at sunset’s gate, Here we hail thee, Alma Mater, Arizona State. —Hopkins-Dresskell MAROON AND GOLD Fight, Devils down the field Fight with your might and don’t ever yield Long may our colors outshine all others Echo from the buttes, Give em’ hell Devils! Cheer, cheer for A-S-U! Fight for the old Maroon For it’s Hail! Hail! The gang’s all here And it’s onward to victory! Students whose names appear in this program have completed degree requirements. -

Hoysala King Ballala Iii (1291-1342 A.D)
FINAL REPORT UGC MINOR RESEARCH PROJECT on LIFE AND ACHIEVEMENTS: HOYSALA KING BALLALA III (1291-1342 A.D) Submitted by DR.N.SAVITHRI Associate Professor Department of History Mallamma Marimallappa Women’s Arts and Commerce College, Mysore-24 Submitted to UNIVERSITY GRANTS COMMISSION South Western Regional Office P.K.Block, Gandhinagar, Bangalore-560009 2017 1 ACKNOWLEDGEMENT First of all, I would like to Express My Gratitude and Indebtedness to University Grants Commission, New Delhi for awarding Minor Research Project in History. My Sincere thanks are due to Sri.Paramashivaiah.S, President of Marimallappa Educational Institutions. I am Grateful to Prof.Panchaksharaswamy.K.N, Honorary Secretary of Marimallappa Educational Institutions. I owe special thanks to Principal Sri.Dhananjaya.Y.D., Vice Principal Prapulla Chandra Kumar.S., Dr.Saraswathi.N., Sri Purushothama.K, Teaching and Non-Teaching Staff, members of Mallamma Marimallappa Women’s College, Mysore. I also thank K.B.Communications, Mysore has taken a lot of strain in computerszing my project work. I am Thankful to the Authorizes of the libraries in Karnataka for giving me permission to consult the necessary documents and books, pertaining to my project work. I thank all the temple guides and curators of minor Hoysala temples like Belur, Halebidu. Somanathapura, Thalkad, Melkote, Hosaholalu, kikkeri, Govindahalli, Nuggehalli, ext…. Several individuals and institution have helped me during the course of this study by generously sharing documents and other reference materials. I am thankful to all of them. Dr.N.Savithri Place: Date: 2 CERTIFICATE I Dr.N. Savithri Certify that the project entitled “LIFE AND ACHIEVEMENTS: HOYSALA KING BALLALA iii (1299-1342 A.D)” sponsored by University Grants Commission New Delhi under Minor Research Project is successfully completed by me. -

Insights Into Abdominal Pregnancy Gwinyai Masukume
WikiJournal of Medicine, 2014, 1 (2) doi: 10.15347/wjm/2014.012 Review Article Insights into abdominal pregnancy Gwinyai Masukume Editor’s note This article provided a great deal of valuable evidence that was not mentioned in the Wikipedia article on ab- dominal pregnancy, and the Wikipedia article has subsequently been expanded with text from this publication. However, because of this purpose, it has never been the aim of this article in itself to be a complete review of the subject, and many aspects of abdominal pregnancy are not included herein. This article also provides an example of how to contribute to Wikimedia projects such as Wikipedia by means of academic publishing. Introduction Risk factors While rare, abdominal pregnancies have a higher Risk factors are similar to tubal pregnancy with sexually chance of maternal mortality, perinatal mortality and transmitted disease playing a major role.[7] However, morbidity compared to normal and ectopic pregnan- about half of those with ectopic pregnancy have no cies, but on occasion a healthy viable infant can be de- known risk factors - known risk factors include damage livered.[1] to the Fallopian tubes from previous surgery or from previous ectopic pregnancy and tobacco smoking.[8] Because tubal, ovarian and broad ligament pregnancies are as difficult to diagnose and treat as abdominal preg- nancies, their exclusion from the most common defini- tion of abdominal pregnancy has been debated.[2] Mechanism Others - in the minority - are of the view that abdominal Typically an abdominal -

Persianism in Antiquity
Oriens et Occidens – Band 25 Franz Steiner Verlag Sonderdruck aus: Persianism in Antiquity Edited by Rolf Strootman and Miguel John Versluys Franz Steiner Verlag, Stuttgart 2017 CONTENTS Acknowledgments . 7 Rolf Strootman & Miguel John Versluys From Culture to Concept: The Reception and Appropriation of Persia in Antiquity . 9 Part I: Persianization, Persomania, Perserie . 33 Albert de Jong Being Iranian in Antiquity (at Home and Abroad) . 35 Margaret C. Miller Quoting ‘Persia’ in Athens . 49 Lloyd Llewellyn-Jones ‘Open Sesame!’ Orientalist Fantasy and the Persian Court in Greek Art 430–330 BCE . 69 Omar Coloru Once were Persians: The Perception of Pre-Islamic Monuments in Iran from the 16th to the 19th Century . 87 Judith A. Lerner Ancient Persianisms in Nineteenth-Century Iran: The Revival of Persepolitan Imagery under the Qajars . 107 David Engels Is there a “Persian High Culture”? Critical Reflections on the Place of Ancient Iran in Oswald Spengler’s Philosophy of History . 121 Part II: The Hellenistic World . 145 Damien Agut-Labordère Persianism through Persianization: The Case of Ptolemaic Egypt . 147 Sonja Plischke Persianism under the early Seleukid Kings? The Royal Title ‘Great King’ . 163 Rolf Strootman Imperial Persianism: Seleukids, Arsakids and Fratarakā . 177 6 Contents Matthew Canepa Rival Images of Iranian Kingship and Persian Identity in Post-Achaemenid Western Asia . 201 Charlotte Lerouge-Cohen Persianism in the Kingdom of Pontic Kappadokia . The Genealogical Claims of the Mithridatids . 223 Bruno Jacobs Tradition oder Fiktion? Die „persischen“ Elemente in den Ausstattungs- programmen Antiochos’ I . von Kommagene . 235 Benedikt Eckhardt Memories of Persian Rule: Constructing History and Ideology in Hasmonean Judea . -

Extra Uterine Pregnancy
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1933 Extra uterine pregnancy Jacob F. Schultz University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Recommended Citation Schultz, Jacob F., "Extra uterine pregnancy" (1933). MD Theses. 290. https://digitalcommons.unmc.edu/mdtheses/290 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. EX! R A - U ! E R I N E PRE G NAN C Y. JACOB F. SCHUL!Z. 480528 1 HISTORY Extra-ute ine pregn_cy was apparently Ul'lkna1'lll to the ancleJllts, theft- bei~ no reference to the su.b~ee't in the works on Greek or Roman meti eiDt. The f'ir st recorded case is that of' A1bucasis, an Arabian ph7s1cian living in Spain about the middle of' tbt eleventh century. He reports a ease whe re he saw parts of' a foetal body escaping from the abdomen of a woman by the process of suppurat ion. This was a case of a long retained secondary abdominal pregnaBcy, and all at the older eases that were reported were of - this type. Al'lother interesting example is that of the lithopedion of sens, Reported by Cordeaus early in the sixteenth century. -

Introduction and Index
Th e Practical Christology of Philoxenos of Mabbug DAVID A. MICHELSON Preview - Copyrighted Material 1 1 Great Clarendon Street, Oxford, OX2 6DP, United Kingdom Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide. Oxford is a registered trade mark of Oxford University Press in the UK and in certain other countries © David A. Michelson 2014 Th e moral rights of the author have been asserted First Edition published in 2014 Impression: 1 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of Oxford University Press, or as expressly permitted by law, by licence or under terms agreed with the appropriate reprographics rights organization. Enquiries concerning reproduction outside the scope of the above should be sent to the Rights Department, Oxford University Press, at the address above You must not circulate this work in any other form and you must impose this same condition on any acquirer Published in the United States of America by Oxford University Press 198 Madison Avenue, New York, NY 10016, United States of America British Library Cataloguing in Publication Data Data available Library of Congress Control Number: 2014940446 ISBN 978–0–19–872296–0 Printed and bound by CPI Group (UK) Ltd, Croydon, CR0 4YY Links to third party websites are provided by Oxford in good faith and for information only. Oxford disclaims any responsibility for the materials contained in any third party website referenced in this work. -
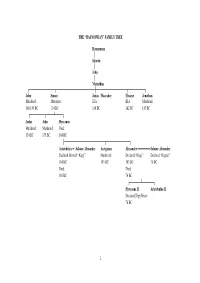
Hasmonean” Family Tree
THE “HASMONEAN” FAMILY TREE Hasmoneus │ Simeon │ John │ Mattathias ┌──────────────┬─────────────────────┼─────────────────┬─────────┐ John Simon Judas Maccabee Eleazar Jonathan Murdered: Murdered: KIA: KIA: Murdered: 160/159 BC 134 BC 160 BC 162 BC 143 BC ┌────────┬────┴────┐ Judas John Hyrcanus Murdered: Murdered: Died: 134 BC 135 BC 104 BC ├──────────────────────┬─────────────┐ Aristobulus ═ Salome Alexander Antigonus Alexander ═══════ Salome Alexander Declared Himself “King”: Murdered: Declared “King”: Declared “Regent”: 104 BC 103 BC 103 BC 76 BC Died: Died: 103 BC 76 BC ┌──────┴──────┐ Hyrcanus II Aristobulus II Declared High Priest: 76 BC 1 THE “HASMONEAN” DYNASTY OF SIMON THE HIGH PRIEST 142 BC Simon, the last of the sons of Mattathias, was declared High Priest & “Ethnarch” (ruler of one’s own ethnic group) of the Jews by Demetrius II, King of the Seleucid Empire. 138 BC After Demetrius II was captured by the Parthians, his brother, Antiochus VII, affirmed Simon’s High Priesthood & requested assistance in dealing with Trypho, a usurper of the Seleucid throne. “King Antiochus to Simon the high priest and ethnarch and to the nation of the Jews, greetings. “Whereas certain scoundrels have gained control of the kingdom of our ancestors, and I intend to lay claim to the kingdom so that I may restore it as it formerly was, and have recruited a host of mercenary troops and have equipped warships, and intend to make a landing in the country so that I may proceed against those who have destroyed our country and those who have devastated many cities in my kingdom, now therefore I confirm to you all the tax remissions that the kings before me have granted you, and a release from all the other payments from which they have released you. -

200 Bc - Ad 400)
ARAM, 13-14 (2001-2002), 171-191 P. ARNAUD 171 BEIRUT: COMMERCE AND TRADE (200 BC - AD 400) PASCAL ARNAUD We know little of Beirut's commerce and trade, and shall probably continue to know little about this matter, despite a lecture given by Mrs Nada Kellas in 19961. In fact, the history of Commerce and Trade relies mainly on both ar- chaeological and epigraphical evidence. As far as archaeological evidence is concerned, one must remember that only artefacts strongly linked with ceram- ics, i.e. vases themselves and any items, carried in amphoras, (predominantly, but not solely, liquids, can give information about the geographical origin, date and nature of such products. The huge quantities of materials brought to the light by recent excavations in Beirut should, one day, provide us with new evi- dence about importations of such products in Beirut, but we will await the complete study of this material, which, until today by no means provided glo- bal statistics valid at the whole town scale. The evidence already published still allows nothing more than mere subjective impressions about the origins of the material. I shall try nevertheless to rely on such impressions about that ma- terial, given that we lack statistics, and that it is impossible to infer from any isolated sherd the existence of permanent trade-routes and commercial flows. The results of such an inquiry would be, at present, worth little if not con- fronted with other evidence. On the other hand, it should be of great interest to identify specific Berytan productions among the finds from other sites in order to map the diffusion area of items produced in Beirut and the surrounding territory. -

Linguistic Study About the Origins of the Aegean Scripts
Anistoriton Journal, vol. 15 (2016-2017) Essays 1 Cretan Hieroglyphics The Ornamental and Ritual Version of the Cretan Protolinear Script The Cretan Hieroglyphic script is conventionally classified as one of the five Aegean scripts, along with Linear-A, Linear-B and the two Cypriot Syllabaries, namely the Cypro-Minoan and the Cypriot Greek Syllabary, the latter ones being regarded as such because of their pictographic and phonetic similarities to the former ones. Cretan Hieroglyphics are encountered in the Aegean Sea area during the 2nd millennium BC. Their relationship to Linear-A is still in dispute, while the conveyed language (or languages) is still considered unknown. The authors argue herein that the Cretan Hieroglyphic script is simply a decorative version of Linear-A (or, more precisely, of the lost Cretan Protolinear script that is the ancestor of all the Aegean scripts) which was used mainly by the seal-makers or for ritual usage. The conveyed language must be a conservative form of Sumerian, as Cretan Hieroglyphic is strictly associated with the original and mainstream Minoan culture and religion – in contrast to Linear-A which was used for several other languages – while the phonetic values of signs have the same Sumerian origin as in Cretan Protolinear. Introduction The three syllabaries that were used in the Aegean area during the 2nd millennium BC were the Cretan Hieroglyphics, Linear-A and Linear-B. The latter conveys Mycenaean Greek, which is the oldest known written form of Greek, encountered after the 15th century BC. Linear-A is still regarded as a direct descendant of the Cretan Hieroglyphics, conveying the unknown language or languages of the Minoans (Davis 2010). -

Philip V and Perseus: the Twilight of Antigonid Macedonia Philip V of Macedonia Was a Shrewd and Effective Leader. He Proved Ev
Philip V and Perseus: The Twilight of Antigonid Macedonia Philip V of Macedonia was a shrewd and effective leader. He proved even more adept than his predecessors at dealing with the Greek city-states, Illyrian invasions, and the other traditional concerns of his kingdom. Unfortunately for him, he was forced to deal with a completely new threat, for which he was unprepared—the rising power of Rome. Philip V and his son and successor Perseus failed in their conflicts with Rome, and ultimately allowed Macedonia to be conquered by the Romans. Since the wars they fought against Rome were recorded by Roman historians, they are known as the Macedonian Wars. Early Life and Reign of Philip V Philip V was the son of Demetrius II, who died in battle when Philip was nine years old. Since the army and nobility were hesitant to trust the kingdom to a child, they made Antigonas Doson regent, and then king. Antigonas honored Philip’s position, and when Antigonas died in 221 BC, Philip ascended smoothly to the throne at the age of seventeen. As the young king of Macedonia, Philip V was eager to prove his abilities. He defeated the Dardians in battle. When hostilities broke out between the two major leagues of Greek cities—the Achaean League and Aetolian League—he sided with Aratus and the Achaean League. Thanks to Philip’s intervention, the Achaeans achieved major victories against the Aetolians, and Aratus became one of Philip’s advisors. First Macedonian War (214–205 BC) In 219 BC, Demetrius of Pharos, the king of Illyria, fled to Philip’s court after being expelled by the Romans. -

Politics and Policy: Rome and Liguria, 200-172 B.C
Politics and policy: Rome and Liguria, 200-172 B.C. Eric Brousseau, Department of History McGill University, Montreal June, 2010 A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Master of Arts. ©Eric Brousseau 2010 i Abstract Stephen Dyson’s The Creation of the Roman Frontier employs various anthropological models to explain the development of Rome’s republican frontiers. His treatment of the Ligurian frontier in the second century BC posits a Ligurian ‘policy’ crafted largely by the Senate and Roman ‘frontier tacticians’ (i.e. consuls). Dyson consciously avoids incorporating the pressures of domestic politics and the dynamics of aristocratic competition. But his insistence that these factors obscure policy continuities is incorrect. Politics determined policy. This thesis deals with the Ligurian frontier from 200 to 172 BC, years in which Roman involvement in the region was most intense. It shows that individual magistrates controlled policy to a much greater extent than Dyson and other scholars have allowed. The interplay between the competing forces of aristocratic competition and Senatorial consensus best explains the continuities and shifts in regional policy. Abstrait The Creation of the Roman Frontier, l’œuvre de Stephen Dyson, utilise plusieurs modèles anthropologiques pour illuminer le développement de la frontière républicaine. Son traitement de la frontière Ligurienne durant la deuxième siècle avant J.-C. postule une ‘politique’ envers les Liguriennes déterminer par le Sénat et les ‘tacticiens de la frontière romain’ (les consuls). Dyson fais exprès de ne pas tenir compte des forces de la politique domestique et la compétition aristocratique. Mais son insistance que ces forces cachent les continuités de la politique Ligurienne est incorrecte.