Regulation of Myelin Structure and Conduction Velocity by Perinodal Astrocytes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Chapter 8 Nervous System
Chapter 8 Nervous System I. Functions A. Sensory Input – stimuli interpreted as touch, taste, temperature, smell, sound, blood pressure, and body position. B. Integration – CNS processes sensory input and initiates responses categorizing into immediate response, memory, or ignore C. Homeostasis – maintains through sensory input and integration by stimulating or inhibiting other systems D. Mental Activity – consciousness, memory, thinking E. Control of Muscles & Glands – controls skeletal muscle and helps control/regulate smooth muscle, cardiac muscle, and glands II. Divisions of the Nervous system – 2 anatomical/main divisions A. CNS (Central Nervous System) – consists of the brain and spinal cord B. PNS (Peripheral Nervous System) – consists of ganglia and nerves outside the brain and spinal cord – has 2 subdivisions 1. Sensory Division (Afferent) – conducts action potentials from PNS toward the CNS (by way of the sensory neurons) for evaluation 2. Motor Division (Efferent) – conducts action potentials from CNS toward the PNS (by way of the motor neurons) creating a response from an effector organ – has 2 subdivisions a. Somatic Motor System – controls skeletal muscle only b. Autonomic System – controls/effects smooth muscle, cardiac muscle, and glands – 2 branches • Sympathetic – accelerator “fight or flight” • Parasympathetic – brake “resting and digesting” * 4 Types of Effector Organs: skeletal muscle, smooth muscle, cardiac muscle, and glands. III. Cells of the Nervous System A. Neurons – receive stimuli and transmit action potentials -

Astrocyte Cell Culture Preparation of Flasks: 1
Astrocyte Cell Culture Preparation of flasks: 1. Coat T75 flask(s) with 1 mg/ml of PureCol (Collagen) overnight 2. Remove solution, rinse flasks with sterile ddH20, set the flasks upright and allow to dry in culture hood for 2 hr Dissection: 1. Dissect P1-P3 pups: Remove brainstem, cerebellum and diencephalons in cold dissection buffer. Peel off meninges and transfer cortex to a 50 ml tube on ice, which contains 20 ml of cold dissection buffer. (Dissect 2 pups for 2 x 106 cells/flask). 2. Carefully pour tissue into a 10 cm dish and gently mince tissue with sterile scissors or razor blade. 3. Transfer tissue to back to 50 ml tube and add 5 ml 1X trypsin and 50 uL DNAse for 25 min at 37ºC. Swirl tube every 5 min. 4. Wash the cortices with Glial Medium twice. 5. Dissociate the tissue by gently triturating the cortices through a 5 ml or 2 ml pipette, followed by a fire-polished Pasteur pipette (3 X 3 triturations). Each time fill pipette with dissociated cells and transfer supernatant to a fresh tube. 6. Dilute cell suspension to 10 ml of Glial Medium, and pass through a 40 uM cell strainer. 7. Spin down the cells at 1700 rpm for 5 min. 8. Re-suspend the cells with 10 ml of Glial Medium, and count. 9. Seed 2 x 106 cells/flask in 15 ml Glial medium. ****(2.0 x 106 cells/flask = 1.33 x 105 cells/ml = 2.67 x 104 cells/cm2)***** 10. Change the medium each of the next two days by aspirating the medium, and then adding back 15 ml of fresh Glial Medium. -

Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3
New Research Neuronal Excitability Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3 Allan T. Gulledge1 and Jaime J. Bravo2 DOI:http://dx.doi.org/10.1523/ENEURO.0085-15.2016 1Department of Physiology and Neurobiology, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire 03756, and 2Thayer School of Engineering at Dartmouth, Hanover, New Hampshire 03755 Visual Abstract In most vertebrate neurons, action potentials are initiated in the axon initial segment (AIS), a specialized region of the axon containing a high density of voltage-gated sodium and potassium channels. It has recently been proposed that neurons use plasticity of AIS length and/or location to regulate their intrinsic excitability. Here we quantify the impact of neuron morphology on AIS plasticity using computational models of simplified and realistic somatodendritic morphologies. In small neurons (e.g., den- tate granule neurons), excitability was highest when the AIS was of intermediate length and located adjacent to the soma. Conversely, neu- rons having larger dendritic trees (e.g., pyramidal neurons) were most excitable when the AIS was longer and/or located away from the soma. For any given somatodendritic morphology, increasing dendritic mem- brane capacitance and/or conductance favored a longer and more distally located AIS. Overall, changes to AIS length, with corresponding changes in total sodium conductance, were far more effective in regulating neuron excitability than were changes in AIS location, while dendritic capacitance had a larger impact on AIS performance than did dendritic conductance. The somatodendritic influence on AIS performance reflects modest soma- to-AIS voltage attenuation combined with neuron size-dependent changes in AIS input resistance, effective membrane time constant, and isolation from somatodendritic capacitance. -

Clustering of Na+ Channels and Node of Ranvier Formation in Remyelinating Axons
The Journal of Neuroscience, January 1995, 15(l): 492503 Clustering of Na+ Channels and Node of Ranvier Formation in Remyelinating Axons Sanja Dugandgija-NovakoviC,’ Adam G. Koszowski,2 S. Rock Levinson,2 and Peter Shragerl ‘Department of Physiology, University of Rochester Medical Center, Rochester, New York 14642 and 2Department of Physiology, University of Colorado Health Sciences Center, Denver, Colorado 80262 Polyclonal antibodies were raised against a well conserved nodal regions(Black et al., 1990). The density of Na+ channels, region of the vertebrate Na+ channel and were affinity pu- in particular, is about 25 times higher at nodesof Ranvier than rified for use in immunocytochemistry. Focal demyelination at internodal sites (Shrager, 1989). There has been vigorous of rat sciatic axons was initiated by an intraneural injection debate over the mechanism of Na+ channel clustering during of lysolecithin and Na+ channel clustering was followed at myelination, particularly with respect to the role of Schwann several stages of myelin removal and repair. At 1 week post- cells, and studies have included both developing nerve and injection axons contained long, fully demyelinated regions. pathological conditions (Ellisman, 1979; Rosenbluth, 1979; Ro- Na+ channel clusters appeared only at heminodes forming senbluth and Blakemore, 1984; Le Beau et al., 1987; England the borders of these zones, and at widely spaced isolated et al., 1990, 1991; Joe and Angelides, 1992, 1993).There remain sites that may represent former nodes of Ranvier. Over the many interesting questions, particularly regarding remodeling next few days proliferating Schwann cells adhered to axons that occurs following myelin disruption. When axons are de- and began to extend processes. -

Myelin Biogenesis Is Associated with Pathological Ultrastructure That Is
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Myelin biogenesis is associated with pathological ultrastructure that 2 is resolved by microglia during development 3 4 5 Minou Djannatian1,2*, Ulrich Weikert3, Shima Safaiyan1,2, Christoph Wrede4, Cassandra 6 Deichsel1,2, Georg Kislinger1,2, Torben Ruhwedel3, Douglas S. Campbell5, Tjakko van Ham6, 7 Bettina Schmid2, Jan Hegermann4, Wiebke Möbius3, Martina Schifferer2,7, Mikael Simons1,2,7* 8 9 1Institute of Neuronal Cell Biology, Technical University Munich, 80802 Munich, Germany 10 2German Center for Neurodegenerative Diseases (DZNE), 81377 Munich, Germany 11 3Max-Planck Institute of Experimental Medicine, 37075 Göttingen, Germany 12 4Institute of Functional and Applied Anatomy, Research Core Unit Electron Microscopy, 13 Hannover Medical School, 30625, Hannover, Germany 14 5Department of Neuronal Remodeling, Graduate School of Pharmaceutical Sciences, Kyoto 15 University, Sakyo-ku, Kyoto 606-8501, Japan. 16 6Department of Clinical Genetics, Erasmus MC, University Medical Center Rotterdam, 3015 CN, 17 the Netherlands 18 7Munich Cluster of Systems Neurology (SyNergy), 81377 Munich, Germany 19 20 *Correspondence: [email protected] or [email protected] 21 Keywords 22 Myelination, degeneration, phagocytosis, microglia, oligodendrocytes, phosphatidylserine 23 24 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -
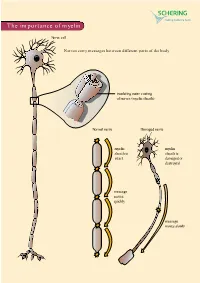
The Importance of Myelin
The importance of myelin Nerve cell Nerves carry messages between different parts of the body insulating outer coating of nerves (myelin sheath) Normal nerve Damaged nerve myelin myelin sheath is sheath is intact damaged or destroyed message moves quickly message moves slowly Nerve cells transmit impulses Nerve cells have a long, thin, flexible fibre that transmits impulses. These impulses are electrical signals that travel along the length of the nerve. Nerve fibres are long to enable impulses to travel between distant parts of the body, such as the spinal cord and leg muscles. Myelin speeds up impulses Most nerve fibres are surrounded by an insulating, fatty sheath called myelin, which acts to speed up impulses. The myelin sheath contains periodic breaks called nodes of Ranvier. By jumping from node to node, the impulse can travel much more quickly than if it had to travel along the entire length of the nerve fibre. Myelinated nerves can transmit a signal at speeds as high as 100 metres per second – as fast as a Formula One racing car. normal damaged nerve nerve Loss of myelin leads to a variety of symptoms If the myelin sheath surrounding nerve fibres is damaged or destroyed, transmission of nerve impulses is slowed or blocked. The impulse now has to flow continuously along the whole nerve fibre – a process that is much slower than jumping from node to node. Loss of myelin can also lead to ‘short-circuiting’ of nerve impulses. An area where myelin has been destroyed is called a lesion or plaque. This slowing and ‘short-circuiting’ of nerve impulses by lesions leads to a variety of symptoms related to nervous system activity. -

How Does an Axon Grow?
Downloaded from genesdev.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW How does an axon grow? Jeffrey L. Goldberg1 Department of Neurobiology, Stanford University School of Medicine, Stanford, California 94305, USA How do axons grow during development, and why do cisions to advance, retract, pause, or turn (Fig. 1). All of they fail to regrow when injured? In the complicated these are potential regulatory sites that could control a mesh of our nervous system, the axon is the information neuron’s ability to elongate or regenerate its axon. superhighway, carrying all of the data we use to sense our environment and carry out behaviors. To wire up our Production of the building blocks, nervous system properly, neurons must elongate their both membrane and cytoplasmic axons during development to reach their targets. This is no simple task, however. The complex morphology of Rapid axon growth requires rapid manufacture and sup- axons and dendrites puts neurons among the most intri- ply of cytoplasm and membrane. Where are the lipid and cate and beautiful cells in the body. Knowledge of how protein building blocks made? It was known from classic neurons extend axons and dendrites, elongate at a par- experiments that axons cut off from the cell bodies of ticular rate, and stop growing at the proper time is criti- adult sensory neurons continue to elongate in culture cal to understanding the development of our nervous (Shaw and Bray 1977), but evidence for local production system, yet the regulation of these processes is poorly of membrane and cytoplasmic elements went lacking for understood. -

The Myelin-Forming Cells of the Nervous System (Oligodendrocytes and Schwann Cells)
The Myelin-Forming Cells of the Nervous System (oligodendrocytes and Schwann cells) Oligodendrocyte Schwann Cell Oligodendrocyte function Saltatory (jumping) nerve conduction Oligodendroglia PMD PMD Saltatory (jumping) nerve conduction Investigating the Myelinogenic Potential of Individual Oligodendrocytes In Vivo Sparse Labeling of Oligodendrocytes CNPase-GFP Variegated expression under the MBP-enhancer Cerebral Cortex Corpus Callosum Cerebral Cortex Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Ant Commissure Corpus Callosum Cerebral Cortex Caudate Putamen Piriform Cortex Corpus Callosum Ant Commissure Characterization of Oligodendrocyte Morphology Cerebral Cortex Corpus Callosum Caudate Putamen Cerebellum Brain Stem Spinal Cord Oligodendrocytes in disease: Cerebral Palsy ! CP major cause of chronic neurological morbidity and mortality in children ! CP incidence now about 3/1000 live births compared to 1/1000 in 1980 when we started intervening for ELBW ! Of all ELBW {gestation 6 mo, Wt. 0.5kg} , 10-15% develop CP ! Prematurely born children prone to white matter injury {WMI}, principle reason for the increase in incidence of CP ! ! 12 Cerebral Palsy Spectrum of white matter injury ! ! Macro Cystic Micro Cystic Gliotic Khwaja and Volpe 2009 13 Rationale for Repair/Remyelination in Multiple Sclerosis Oligodendrocyte specification oligodendrocytes specified from the pMN after MNs - a ventral source of oligodendrocytes -

NEURAL CONNECTIONS: Some You Use, Some You Lose
NEURAL CONNECTIONS: Some You Use, Some You Lose by JOHN T. BRUER SOURCE: Phi Delta Kappan 81 no4 264-77 D 1999 . The magazine publisher is the copyright holder of this article and it is reproduced with permission. Further reproduction of this article in violation of the copyright is prohibited JOHN T. BRUER is president of the James S. McDonnell Foundation, St. Louis. This article is adapted from his new book, The Myth of the First Three Years (Free Press, 1999), and is reprinted by arrangement with The Free Press, a division of Simon Schuster Inc. ©1999, John T. Bruer . OVER 20 YEARS AGO, neuroscientists discovered that humans and other animals experience a rapid increase in brain connectivity -- an exuberant burst of synapse formation -- early in development. They have studied this process most carefully in the brain's outer layer, or cortex, which is essentially our gray matter. In these studies, neuroscientists have documented that over our life spans the number of synapses per unit area or unit volume of cortical tissue changes, as does the number of synapses per neuron. Neuroscientists refer to the number of synapses per unit of cortical tissue as the brain's synaptic density. Over our lifetimes, our brain's synaptic density changes in an interesting, patterned way. This pattern of synaptic change and what it might mean is the first neurobiological strand of the Myth of the First Three Years. (The second strand of the Myth deals with the notion of critical periods, and the third takes up the matter of enriched, or complex, environments.) Popular discussions of the new brain science trade heavily on what happens to synapses during infancy and childhood. -

Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Disease
MOG ANTIBODY DISEASE Myelin Oligodendrocyte Glycoprotein (MOG) Antibody-Associated Encephalomyelitis WHAT IS MOG ANTIBODY-ASSOCIATED DEMYELINATION? Myelin oligodendrocyte glycoprotein (MOG) antibody-associated demyelination is an immune-mediated inflammatory process that affects the central nervous system (brain and spinal cord). MOG is a protein that exists on the outer surface of cells that create myelin (an insulating layer around nerve fibers). In a small number of patients, an initial episode of inflammation due to MOG antibodies may be the first manifestation of multiple sclerosis (MS). Most patients may only experience one episode of inflammation, but repeated episodes of central nervous system demyelination can occur in some cases. WHAT ARE THE SYMPTOMS? • Optic neuritis (inflammation of the optic nerve(s)) may be a symptom of MOG antibody- associated demyelination, which may result in painful loss of vision in one or both eyes. • Transverse myelitis (inflammation of the spinal cord) may cause a variety of symptoms that include: o Abnormal sensations. Numbness, tingling, coldness or burning in the arms and/or legs. Some are especially sensitive to the light touch of clothing or to extreme heat or cold. You may feel as if something is tightly wrapping the skin of your chest, abdomen or legs. o Weakness in your arms or legs. Some people notice that they're stumbling or dragging one foot, or heaviness in the legs. Others may develop severe weakness or even total paralysis. o Bladder and bowel problems. This may include needing to urinate more frequently, urinary incontinence, difficulty urinating and constipation. • Acute disseminated encephalomyelitis (ADEM) may cause loss of vision, weakness, numbness, and loss of balance, and altered mental status.