The Effects of Normal Aging on Myelin and Nerve Fibers: a Review∗
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Visual Fixation Development in Children
Graefe’s Arch Clin Exp Ophthalmol (2007) 245:1659–1665 DOI 10.1007/s00417-007-0585-6 CLINICAL INVESTIGATION Visual fixation development in children Eva Aring & Marita Andersson Grönlund & Ann Hellström & Jan Ygge Received: 12 December 2006 /Revised: 2 March 2007 /Accepted: 31 March 2007 / Published online: 24 April 2007 # Springer-Verlag 2007 Abstract there were no significant differences with regard to gender Background The ability to keep steady fixation on a target or laterality in any of the investigated variables. No is one of several aspects of good visual function. However, nystagmus was observed. there are few reports on visual fixation during childhood in Conclusion This study establishes values for visual fixation healthy children. behaviour in a non-clinical population aged 4–15 years, Methods An infrared eye-tracking device (Orbit) was used which can be used for identifying children with fixation to analyse binocular fixation behaviour in 135 non-clinical abnormalities. participants aged 4–15 years. The children wore goggles and their heads were restrained using a chin and forehead Keywords Blinks . Drifts . Intruding saccades . rest, while binocularly fixating a stationary target for 20 s. Centre of gravity Results The density of fixations around the centre of gravity increased with increasing age (p<0.01), and the time of fixation without intruding movements increased Introduction with increasing age (p=0.02), while intruding saccades decreased with increasing age (p<0.01). The number of The ability to visually fixate a target is one of several blinks and drifts did not differ between 4 and 15 years, and aspects of good visual function [1]. -
Final Version
REWARD LIST ‐ RANK C JCR 2018 ‐CiteScore 2018 WOS Edition/ Scopus Main Rank Subject Category Journal Title ISSN Subject Area code IEEE TRANSACTIONS ON ULTRASONICS SCIE ACOUSTICS 08853010 C FERROELECTRICS AND FREQUENCY SCIE ACOUSTICS JOURNAL OF VIBRATION AND CONTROL 10775463 C SCIE AGRICULTURAL ECONOMICS & POLICY JOURNAL OF AGRICULTURAL ECONOMICS 0021857X C SCIE AGRICULTURAL ENGINEERING BIOMASS & BIOENERGY 09619534 C SCIE AGRICULTURE, DAIRY & ANIMAL SCIENCE ANIMAL REPRODUCTION SCIENCE 03784320 C SCIE AGRICULTURE, DAIRY & ANIMAL SCIENCE APPLIED ANIMAL BEHAVIOUR SCIENCE 01681591 C SCIE AGRICULTURE, DAIRY & ANIMAL SCIENCE ARCHIVES OF ANIMAL NUTRITION 1745039X C JOURNAL OF ANIMAL PHYSIOLOGY AND SCIE AGRICULTURE, DAIRY & ANIMAL SCIENCE 09312439 C ANIMAL NUTRITION SCIE AGRICULTURE, DAIRY & ANIMAL SCIENCE Animals 20762615 C SCIE AGRICULTURE, MULTIDISCIPLINARY Journal of Land Use Science 1747423X C RENEWABLE AGRICULTURE AND FOOD SCIE AGRICULTURE, MULTIDISCIPLINARY 17421705 C SYSTEMS SCIE AGRICULTURE, MULTIDISCIPLINARY ANNALS OF APPLIED BIOLOGY 00034746 C SCIE AGRICULTURE, MULTIDISCIPLINARY CALIFORNIA AGRICULTURE 00080845 C SCIE AGRONOMY PLANT PATHOLOGY 00320862 C SCIE AGRONOMY IRRIGATION SCIENCE 03427188 C SCIE AGRONOMY Rice Science 16726308 C SCIE AGRONOMY Agronomy‐Basel 20734395 C SCIE AGRONOMY CROP PROTECTION 02612194 C SCIE AGRONOMY EXPERIMENTAL AGRICULTURE 00144797 C JOURNAL OF PLANT NUTRITION AND SOIL SCIE AGRONOMY 14368730 C SCIENCE SCIE ALLERGY CONTACT DERMATITIS 01051873 C SCIE ALLERGY Allergy Asthma & Immunology Research 20927355 C Advances -

Myelin Biogenesis Is Associated with Pathological Ultrastructure That Is
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Myelin biogenesis is associated with pathological ultrastructure that 2 is resolved by microglia during development 3 4 5 Minou Djannatian1,2*, Ulrich Weikert3, Shima Safaiyan1,2, Christoph Wrede4, Cassandra 6 Deichsel1,2, Georg Kislinger1,2, Torben Ruhwedel3, Douglas S. Campbell5, Tjakko van Ham6, 7 Bettina Schmid2, Jan Hegermann4, Wiebke Möbius3, Martina Schifferer2,7, Mikael Simons1,2,7* 8 9 1Institute of Neuronal Cell Biology, Technical University Munich, 80802 Munich, Germany 10 2German Center for Neurodegenerative Diseases (DZNE), 81377 Munich, Germany 11 3Max-Planck Institute of Experimental Medicine, 37075 Göttingen, Germany 12 4Institute of Functional and Applied Anatomy, Research Core Unit Electron Microscopy, 13 Hannover Medical School, 30625, Hannover, Germany 14 5Department of Neuronal Remodeling, Graduate School of Pharmaceutical Sciences, Kyoto 15 University, Sakyo-ku, Kyoto 606-8501, Japan. 16 6Department of Clinical Genetics, Erasmus MC, University Medical Center Rotterdam, 3015 CN, 17 the Netherlands 18 7Munich Cluster of Systems Neurology (SyNergy), 81377 Munich, Germany 19 20 *Correspondence: [email protected] or [email protected] 21 Keywords 22 Myelination, degeneration, phagocytosis, microglia, oligodendrocytes, phosphatidylserine 23 24 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -
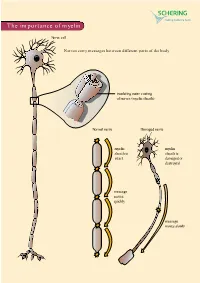
The Importance of Myelin
The importance of myelin Nerve cell Nerves carry messages between different parts of the body insulating outer coating of nerves (myelin sheath) Normal nerve Damaged nerve myelin myelin sheath is sheath is intact damaged or destroyed message moves quickly message moves slowly Nerve cells transmit impulses Nerve cells have a long, thin, flexible fibre that transmits impulses. These impulses are electrical signals that travel along the length of the nerve. Nerve fibres are long to enable impulses to travel between distant parts of the body, such as the spinal cord and leg muscles. Myelin speeds up impulses Most nerve fibres are surrounded by an insulating, fatty sheath called myelin, which acts to speed up impulses. The myelin sheath contains periodic breaks called nodes of Ranvier. By jumping from node to node, the impulse can travel much more quickly than if it had to travel along the entire length of the nerve fibre. Myelinated nerves can transmit a signal at speeds as high as 100 metres per second – as fast as a Formula One racing car. normal damaged nerve nerve Loss of myelin leads to a variety of symptoms If the myelin sheath surrounding nerve fibres is damaged or destroyed, transmission of nerve impulses is slowed or blocked. The impulse now has to flow continuously along the whole nerve fibre – a process that is much slower than jumping from node to node. Loss of myelin can also lead to ‘short-circuiting’ of nerve impulses. An area where myelin has been destroyed is called a lesion or plaque. This slowing and ‘short-circuiting’ of nerve impulses by lesions leads to a variety of symptoms related to nervous system activity. -

Localization of Borrelia Burgdorferi in the Nervous System And
0023-6837/00/8007-1043$03.00/0 LABORATORY INVESTIGATION Vol. 80, No. 7, p. 1043, 2000 Copyright © 2000 by The United States and Canadian Academy of Pathology, Inc. Printed in U.S.A. Localization of Borrelia Burgdorferi in the Nervous System and Other Organs in a Nonhuman Primate Model of Lyme Disease Diego Cadavid, Tim O’Neill, Henry Schaefer, and Andrew R. Pachner Department of Neuroscience (DC, ARP), University of Medicine and Dentistry of New Jersey-New Jersey Medical School, Newark, New Jersey; and the Registry of Comparative Pathology (TO) and the Department of Neuropa- thology (DC), Armed Forces Institute of Pathology, and the Department of Neurology (DC, HS, ARP), Georgetown University Medical Center, Washington, DC SUMMARY: Lyme borreliosis is caused by infection with the spirochete Borrelia burgdorferi. Nonhuman primates inoculated with the N40 strain of B. burgdorferi develop infection of multiple tissues, including the central (CNS) and peripheral nervous system. In immunocompetent nonhuman primates, spirochetes are present in low numbers in tissues. For this reason, it has been difficult to study their localization and changes in expression of surface proteins. To further investigate this, we inoculated four immunosuppressed adult Macaca mulatta with 1 million spirochetes of the N40 strain of B. burgdorferi, and compared them with three infected immunocompetent animals and two uninfected controls. The brain, spinal cord, peripheral nerves, skeletal muscle, heart, and bladder were obtained at necropsy 4 months later. The spirochetal tissue load was first studied by polymerase chain reaction (PCR)-ELISA of the outer surface protein A (ospA) gene. Immunohistochemistry was used to study the localization and numbers of spirochetes in tissues and the expression of spirochetal proteins and to characterize the inflammatory response. -

An Adaptive Algorithm for Fixation, Saccade, and Glissade Detection in Eyetracking Data
Behavior Research Methods 2010, 42 (1), 188-204 doi:10.3758/BRM.42.1.188 An adaptive algorithm for fixation, saccade, and glissade detection in eyetracking data MARCUS NYSTRÖM AND KENNETH HOLMQVIST Lund University, Lund, Sweden Event detection is used to classify recorded gaze points into periods of fixation, saccade, smooth pursuit, blink, and noise. Although there is an overall consensus that current algorithms for event detection have serious flaws and that a de facto standard for event detection does not exist, surprisingly little work has been done to remedy this problem. We suggest a new velocity-based algorithm that takes several of the previously known limitations into account. Most important, the new algorithm identifies so-called glissades, a wobbling move- ment at the end of many saccades, as a separate class of eye movements. Part of the solution involves designing an adaptive velocity threshold that makes the event detection less sensitive to variations in noise level and the algorithm settings-free for the user. We demonstrate the performance of the new algorithm on eye movements recorded during reading and scene perception and compare it with two of the most commonly used algorithms today. Results show that, unlike the currently used algorithms, fixations, saccades, and glissades are robustly identified by the new algorithm. Using this algorithm, we found that glissades occur in about half of the sac- cades, during both reading and scene perception, and that they have an average duration close to 24 msec. Due to the high prevalence and long durations of glissades, we argue that researchers must actively choose whether to assign the glissades to saccades or fixations; the choice affects dependent variables such as fixation and sac- cade duration significantly. -

Regulation of Myelin Structure and Conduction Velocity by Perinodal Astrocytes
Correction NEUROSCIENCE Correction for “Regulation of myelin structure and conduc- tion velocity by perinodal astrocytes,” by Dipankar J. Dutta, Dong Ho Woo, Philip R. Lee, Sinisa Pajevic, Olena Bukalo, William C. Huffman, Hiroaki Wake, Peter J. Basser, Shahriar SheikhBahaei, Vanja Lazarevic, Jeffrey C. Smith, and R. Douglas Fields, which was first published October 29, 2018; 10.1073/ pnas.1811013115 (Proc. Natl. Acad. Sci. U.S.A. 115,11832–11837). The authors note that the following statement should be added to the Acknowledgments: “We acknowledge Dr. Hae Ung Lee for preliminary experiments that informed the ultimate experimental approach.” Published under the PNAS license. Published online June 10, 2019. www.pnas.org/cgi/doi/10.1073/pnas.1908361116 12574 | PNAS | June 18, 2019 | vol. 116 | no. 25 www.pnas.org Downloaded by guest on October 2, 2021 Regulation of myelin structure and conduction velocity by perinodal astrocytes Dipankar J. Duttaa,b, Dong Ho Wooa, Philip R. Leea, Sinisa Pajevicc, Olena Bukaloa, William C. Huffmana, Hiroaki Wakea, Peter J. Basserd, Shahriar SheikhBahaeie, Vanja Lazarevicf, Jeffrey C. Smithe, and R. Douglas Fieldsa,1 aSection on Nervous System Development and Plasticity, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892; bThe Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817; cMathematical and Statistical Computing Laboratory, Office of Intramural Research, Center for Information -

The Myelin-Forming Cells of the Nervous System (Oligodendrocytes and Schwann Cells)
The Myelin-Forming Cells of the Nervous System (oligodendrocytes and Schwann cells) Oligodendrocyte Schwann Cell Oligodendrocyte function Saltatory (jumping) nerve conduction Oligodendroglia PMD PMD Saltatory (jumping) nerve conduction Investigating the Myelinogenic Potential of Individual Oligodendrocytes In Vivo Sparse Labeling of Oligodendrocytes CNPase-GFP Variegated expression under the MBP-enhancer Cerebral Cortex Corpus Callosum Cerebral Cortex Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Ant Commissure Corpus Callosum Cerebral Cortex Caudate Putamen Piriform Cortex Corpus Callosum Ant Commissure Characterization of Oligodendrocyte Morphology Cerebral Cortex Corpus Callosum Caudate Putamen Cerebellum Brain Stem Spinal Cord Oligodendrocytes in disease: Cerebral Palsy ! CP major cause of chronic neurological morbidity and mortality in children ! CP incidence now about 3/1000 live births compared to 1/1000 in 1980 when we started intervening for ELBW ! Of all ELBW {gestation 6 mo, Wt. 0.5kg} , 10-15% develop CP ! Prematurely born children prone to white matter injury {WMI}, principle reason for the increase in incidence of CP ! ! 12 Cerebral Palsy Spectrum of white matter injury ! ! Macro Cystic Micro Cystic Gliotic Khwaja and Volpe 2009 13 Rationale for Repair/Remyelination in Multiple Sclerosis Oligodendrocyte specification oligodendrocytes specified from the pMN after MNs - a ventral source of oligodendrocytes -

SCIENCE CITATION INDEX EXPANDED - JOURNAL LIST Total Journals: 8631
SCIENCE CITATION INDEX EXPANDED - JOURNAL LIST Total journals: 8631 1. 4OR-A QUARTERLY JOURNAL OF OPERATIONS RESEARCH 2. AAPG BULLETIN 3. AAPS JOURNAL 4. AAPS PHARMSCITECH 5. AATCC REVIEW 6. ABDOMINAL IMAGING 7. ABHANDLUNGEN AUS DEM MATHEMATISCHEN SEMINAR DER UNIVERSITAT HAMBURG 8. ABSTRACT AND APPLIED ANALYSIS 9. ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY 10. ACADEMIC EMERGENCY MEDICINE 11. ACADEMIC MEDICINE 12. ACADEMIC PEDIATRICS 13. ACADEMIC RADIOLOGY 14. ACCOUNTABILITY IN RESEARCH-POLICIES AND QUALITY ASSURANCE 15. ACCOUNTS OF CHEMICAL RESEARCH 16. ACCREDITATION AND QUALITY ASSURANCE 17. ACI MATERIALS JOURNAL 18. ACI STRUCTURAL JOURNAL 19. ACM COMPUTING SURVEYS 20. ACM JOURNAL ON EMERGING TECHNOLOGIES IN COMPUTING SYSTEMS 21. ACM SIGCOMM COMPUTER COMMUNICATION REVIEW 22. ACM SIGPLAN NOTICES 23. ACM TRANSACTIONS ON ALGORITHMS 24. ACM TRANSACTIONS ON APPLIED PERCEPTION 25. ACM TRANSACTIONS ON ARCHITECTURE AND CODE OPTIMIZATION 26. ACM TRANSACTIONS ON AUTONOMOUS AND ADAPTIVE SYSTEMS 27. ACM TRANSACTIONS ON COMPUTATIONAL LOGIC 28. ACM TRANSACTIONS ON COMPUTER SYSTEMS 29. ACM TRANSACTIONS ON COMPUTER-HUMAN INTERACTION 30. ACM TRANSACTIONS ON DATABASE SYSTEMS 31. ACM TRANSACTIONS ON DESIGN AUTOMATION OF ELECTRONIC SYSTEMS 32. ACM TRANSACTIONS ON EMBEDDED COMPUTING SYSTEMS 33. ACM TRANSACTIONS ON GRAPHICS 34. ACM TRANSACTIONS ON INFORMATION AND SYSTEM SECURITY 35. ACM TRANSACTIONS ON INFORMATION SYSTEMS 36. ACM TRANSACTIONS ON INTELLIGENT SYSTEMS AND TECHNOLOGY 37. ACM TRANSACTIONS ON INTERNET TECHNOLOGY 38. ACM TRANSACTIONS ON KNOWLEDGE DISCOVERY FROM DATA 39. ACM TRANSACTIONS ON MATHEMATICAL SOFTWARE 40. ACM TRANSACTIONS ON MODELING AND COMPUTER SIMULATION 41. ACM TRANSACTIONS ON MULTIMEDIA COMPUTING COMMUNICATIONS AND APPLICATIONS 42. ACM TRANSACTIONS ON PROGRAMMING LANGUAGES AND SYSTEMS 43. ACM TRANSACTIONS ON RECONFIGURABLE TECHNOLOGY AND SYSTEMS 44. -

Blinks, Saccades, and Fixation Pauses During Vigilance Task Office of Aviation Medicine Washington, D.C
DOT/FAAIAM-94/26 Blinks, Saccades, and Fixation Pauses During Vigilance Task Office of Aviation Medicine Washington, D.C. 20591 Performance: I. Time on Task Accesion For John A. Stern NTIS CRA&I Donna Boyer DTIC TAB Department of Psychology Unannounced W ashington U niversity Justification .................................. St. Louis, MO 63130-4899 By ...-. -...-.-................................... David Schroeder Distribution I Mark Touchstone Availability Codes FAA Civil Aeromedical Institute Avail and/or P.O. Box 25082 Dist Special Oklahoma City, OK 73125 Nikolai Stoliarov R4 State Scientific Research Institute for Civil Aviation Volocolamskoe Hwy, 26 Moscow 123182 Russia D • ELECTE December 1994 1 Am 9 1995 Final Report This document is available to the public through the National Technical Information Service, Springfield, Virginia 22161. U.S. Department of Transportation Federal Aviation Administration 19950104 027 NOTICE This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The United States Government assumes no liability for the contents or use thereof. Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT/FAA/AM-94/261 4. Title and Subtitle 5. Report Date Blinks, Saccades, and Fixation Pauses During Vigilance Task December 1994 Performance: I. Time on Task 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. J.A. Stern, D. Boyer, D.J. Schroeder, R.M. Touchstone, N. Stoliarov 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Department of Psychology Human Resources Research Division Washington University FAA Civil Aeromedical Institute St. Louis, MO 63130-4899 Oklahoma City, OK 73125 11. -

Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Disease
MOG ANTIBODY DISEASE Myelin Oligodendrocyte Glycoprotein (MOG) Antibody-Associated Encephalomyelitis WHAT IS MOG ANTIBODY-ASSOCIATED DEMYELINATION? Myelin oligodendrocyte glycoprotein (MOG) antibody-associated demyelination is an immune-mediated inflammatory process that affects the central nervous system (brain and spinal cord). MOG is a protein that exists on the outer surface of cells that create myelin (an insulating layer around nerve fibers). In a small number of patients, an initial episode of inflammation due to MOG antibodies may be the first manifestation of multiple sclerosis (MS). Most patients may only experience one episode of inflammation, but repeated episodes of central nervous system demyelination can occur in some cases. WHAT ARE THE SYMPTOMS? • Optic neuritis (inflammation of the optic nerve(s)) may be a symptom of MOG antibody- associated demyelination, which may result in painful loss of vision in one or both eyes. • Transverse myelitis (inflammation of the spinal cord) may cause a variety of symptoms that include: o Abnormal sensations. Numbness, tingling, coldness or burning in the arms and/or legs. Some are especially sensitive to the light touch of clothing or to extreme heat or cold. You may feel as if something is tightly wrapping the skin of your chest, abdomen or legs. o Weakness in your arms or legs. Some people notice that they're stumbling or dragging one foot, or heaviness in the legs. Others may develop severe weakness or even total paralysis. o Bladder and bowel problems. This may include needing to urinate more frequently, urinary incontinence, difficulty urinating and constipation. • Acute disseminated encephalomyelitis (ADEM) may cause loss of vision, weakness, numbness, and loss of balance, and altered mental status. -

Jennifer I. Luebke 1 Curriculum Vitae Jennifer I. Luebke, Ph.D. Associate
Jennifer I. Luebke Curriculum Vitae Jennifer I. Luebke, Ph.D. Associate Professor of Anatomy & Neurobiology Associate Professor of Psychiatry Director, Laboratory of Cellular Neurobiology Boston University School of Medicine 85 East Newton Street, M949 Boston, Massachusetts 02118 Voice: 617-638-4930 Email: [email protected] Education and Employment History: 1980-1984: B.S. (Biology) Randolph-Macon College, Ashland, Virginia 1984-1986: Laboratory Technician (Laboratory of Cell Biology and Genetics, NIDDK) National Institutes of Health, Bethesda, Maryland 1986-1990: Ph.D. Student (Anatomy & Neurobiology), Boston University School of Medicine, Boston, Massachusetts (Linda L. Wright, mentor) 1990-1992: Postdoctoral Fellow, Department of Psychiatry, Harvard Medical School, Boston, Massachusetts (Robert W. McCarley and Robert W. Greene mentors) 1992-1995: Postdoctoral Fellow, Department of Physiology, Tufts University Medical School, Boston, Massachusetts (Kathleen Dunlap, mentor) 1995-2003: Assistant Professor, Department of Anatomy & Neurobiology and Department of Psychiatry, Boston University School of Medicine, Boston, Massachusetts 2004-Present: Associate Professor, Department of Anatomy & Neurobiology and Department of Psychiatry, Boston University School of Medicine, Boston, Massachusetts 2010-Present: Adjunct Associate Professor, Department of Neuroscience, Mount Sinai School of Medicine, New York, New York Research Summary: Research is directed toward understanding alterations in the structure and function of individual cortical pyramidal cells in a rhesus monkey model of normal aging and in transgenic mouse models of neurodegenerative disease. Using whole-cell patch-clamp methods and ultra-high resolution confocal microscopy, Dr. Luebke has demonstrated marked alterations in action potential firing patterns (and underlying ionic currents), glutamatergic and GABAergic synaptic response properties and detailed dendritic and spine architecture in cortical pyramidal cells both in normal aging and in mouse models of neurodegenerative disease.