Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Consensus Statement on Immune Modulation in Multiple Sclerosis
[Downloaded free from http://www.annalsofian.org on Monday, June 8, 2020, IP: 202.164.42.67] View Point Consensus Statement On Immune Modulation in Multiple Sclerosis and Related Disorders During the COVID‑19 Pandemic: Expert Group on Behalf of the Indian Academy of Neurology Rohit Bhatia, M. V. Padma Srivastava, Dheeraj Khurana1, Lekha Pandit2, Thomas Mathew3, Salil Gupta4, Netravathi M5, Sruthi S. Nair6, Gagandeep Singh7, Bhim S. Singhal8 Department of Neurology, All India Institute of Medical Sciences, New Delhi, 1Department of Neurology, Postgraduate Institute of Medical Education and Research, Chandigarh, 2Department of Neurology, K.S. Hegde Medical Academy, Mangalore, Karnataka, 3Department of Neurology, St John’s Medical College, Bangalore, Karnataka, 4Department of Neurology, Command Hospital Air Force, Bangalore, Karnataka, 5Department of Neurology, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, 6Department of Neurology, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, 7Department of Neurology, Dayanand Medical College and Hospital, Ludhiana, Punjab, 8Department of Neurology, Bombay Hospital, Mumbai, Maharashtra, India Abstract Knowledge related to SARS-CoV-2 or 2019 novel coronavirus (2019-nCoV) is still emerging and rapidly evolving. We know little about the effects of this novel coronavirus on various body systems and its behaviour among patients with underlying neurological conditions, especially those on immunomodulatory medications. The aim of the present consensus expert opinion document is to appraise the potential concerns when managing our patients with underlying CNS autoimmune demyelinating disorders during the current COVID-19 pandemic. Keywords: COVID 19, disease modifying therapy, immunotherapy, MOG, MS, NMOSD INTRODUCTION cough, myalgia and headache with acute respiratory distress syndrome (ARDS) being the most frequent complication, In December 2019, health authorities recognized an outbreak inducing mortality in six (15%) patients. -

SERIES #3 Mlet's Gaoin Gmentum a Collaborative Series Brought to You by the Sumaira Foundation for NMO and the MOG Project ACUTE VS
SERIES #3 MLet's gaOin Gmentum a collaborative series brought to you by The Sumaira Foundation for NMO and The MOG Project ACUTE VS. PREVENTIVE TREATMENTS: What's the difference? ACUTE PREVENTATIVE Acute attacks are suspected when Long-term treatment focused on symptoms last 24 hours or more. modification of the immune system to Medical tests are used to confirm the prevent relapses. attack. To limit overtreatment: usually Short-term treatment focused on recommend to hold preventive therapy to decreasing inflammation (steroids) and recurrent disease (but may consider it antibody removal (IVIG, PLEX). after a single severe attack). Typically started immediately after initial Many patients are on preventive presentation to avoid long-term damage. medications for years. Duration of treatment should be individualized to each patient. MOG antibody titers can decrease with treatment but this may not indicate monophasic disease. The link between MOG antibody titers, treatment and recurring relapses remains uncertain. COMMON TREATMENTS FOR MOG-AD IV STEROIDS S T N E M T A E ORAL R T IVIG A E STEROIDS T U C A PLEX COMMON TREATMENTS FOR MOG-AD S T IVIG N E M T A E R T E azathioprine V mycophenolate I T (CellCept) P (Imuran) A T N E V E R P rituximab (Rituxan) INTRAVENOUS (IV) STEROIDS (METHYLPREDNISOLONE) Acute Treatments HOW IT WORKS WHAT TO EXPECT Part of the drug class corticosteroids. In some cases, patients will be hospitalized for the course of their Acts as an anti-inflammatory agent to treatment, others receive infusion via outpatient services. slow the body’s response to injury or Can address symptoms as early as 2-3 days however recovery disease, including reducing swelling from damage varies. -

Diagnostic Considerations in Acute Disseminated Encephalomyelitis and the Interface with MOG Antibody
Review Article Diagnostic Considerations in Acute Disseminated Encephalomyelitis and the Interface with MOG Antibody Jonathan D. Santoro1,2,3,4 Tanuja Chitnis1,2 1 Department of Neurology, Massachusetts General Hospital, Boston, Address for correspondence Jonathan D. Santoro, MD, Department of Massachusetts, United States Neurology, Massachusetts General Hospital, 55 Fruit Street, ACC 708, 2 Department of Neurology, Harvard Medical School, Boston, Boston, MA 02114, United States (e-mail: [email protected]). Massachusetts, United States 3 Department of Neurology, Children’s Hospital of Los Angeles, Los Angeles, California, United States 4 Keck School of Medicine, University of Southern California, Los Angeles, California, United States Neuropediatrics Abstract Acute disseminated encephalomyelitis (ADEM) is a common yet clinically heterogenous syndrome characterized by encephalopathy, focal neurologic findings, and abnormal Keywords neuroimaging. Differentiating ADEM from other demyelinating disorders of childhood ► ADEM can be difficult and appropriate interpretation of the historical, clinical, and neurodiag- ► acute disseminated nostic components of a patient’s presentation is critical. Myelin oligodendrocyte glycopro- encephalomyelitis tein (MOG) antibody-associated diseases are a recently recognized set of disorders, which ► diagnosis include ADEM presentations, among other phenotypes. This review article discusses the ► treatment clinical diagnosis, differential diagnosis, interpretation of data, and treatment/prognosis of ► MOG antibody this unique syndrome with distinctive review of the spectrum of MOG antibodies. Introduction activated T cells recruits additional immune cells to the CNS leading to primary injury of white matter.7 Although more Acute disseminated encephalomyelitis (ADEM) is an immune- nuanced than described, for the scope of this review, the mediated disorder of the central nervous system (CNS), affect- authors will focus on the clinical aspects of the disease and ing both children and adults. -

Myelin Biogenesis Is Associated with Pathological Ultrastructure That Is
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Myelin biogenesis is associated with pathological ultrastructure that 2 is resolved by microglia during development 3 4 5 Minou Djannatian1,2*, Ulrich Weikert3, Shima Safaiyan1,2, Christoph Wrede4, Cassandra 6 Deichsel1,2, Georg Kislinger1,2, Torben Ruhwedel3, Douglas S. Campbell5, Tjakko van Ham6, 7 Bettina Schmid2, Jan Hegermann4, Wiebke Möbius3, Martina Schifferer2,7, Mikael Simons1,2,7* 8 9 1Institute of Neuronal Cell Biology, Technical University Munich, 80802 Munich, Germany 10 2German Center for Neurodegenerative Diseases (DZNE), 81377 Munich, Germany 11 3Max-Planck Institute of Experimental Medicine, 37075 Göttingen, Germany 12 4Institute of Functional and Applied Anatomy, Research Core Unit Electron Microscopy, 13 Hannover Medical School, 30625, Hannover, Germany 14 5Department of Neuronal Remodeling, Graduate School of Pharmaceutical Sciences, Kyoto 15 University, Sakyo-ku, Kyoto 606-8501, Japan. 16 6Department of Clinical Genetics, Erasmus MC, University Medical Center Rotterdam, 3015 CN, 17 the Netherlands 18 7Munich Cluster of Systems Neurology (SyNergy), 81377 Munich, Germany 19 20 *Correspondence: [email protected] or [email protected] 21 Keywords 22 Myelination, degeneration, phagocytosis, microglia, oligodendrocytes, phosphatidylserine 23 24 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.02.429485; this version posted February 4, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -
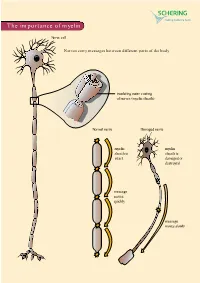
The Importance of Myelin
The importance of myelin Nerve cell Nerves carry messages between different parts of the body insulating outer coating of nerves (myelin sheath) Normal nerve Damaged nerve myelin myelin sheath is sheath is intact damaged or destroyed message moves quickly message moves slowly Nerve cells transmit impulses Nerve cells have a long, thin, flexible fibre that transmits impulses. These impulses are electrical signals that travel along the length of the nerve. Nerve fibres are long to enable impulses to travel between distant parts of the body, such as the spinal cord and leg muscles. Myelin speeds up impulses Most nerve fibres are surrounded by an insulating, fatty sheath called myelin, which acts to speed up impulses. The myelin sheath contains periodic breaks called nodes of Ranvier. By jumping from node to node, the impulse can travel much more quickly than if it had to travel along the entire length of the nerve fibre. Myelinated nerves can transmit a signal at speeds as high as 100 metres per second – as fast as a Formula One racing car. normal damaged nerve nerve Loss of myelin leads to a variety of symptoms If the myelin sheath surrounding nerve fibres is damaged or destroyed, transmission of nerve impulses is slowed or blocked. The impulse now has to flow continuously along the whole nerve fibre – a process that is much slower than jumping from node to node. Loss of myelin can also lead to ‘short-circuiting’ of nerve impulses. An area where myelin has been destroyed is called a lesion or plaque. This slowing and ‘short-circuiting’ of nerve impulses by lesions leads to a variety of symptoms related to nervous system activity. -

MOG Antibody Positive Optic Perineuritis: an Observational Study in Han Chinese
MOG Antibody Positive Optic Perineuritis: an Observational Study in Han Chinese CHAO MENG Beijing Tongren Hospital https://orcid.org/0000-0002-9011-6693 Jia-wei Wang Beijing Tongren Hospital Lei Liu Beijing Tongren Hospital Wen-jing Wei Beijing Tongren Hospital Jian-hua Tao Beijing Tongren Hospital Yong Li Beijing Tongren Hospital Qing-lin Yang Beijing Tongren Hospital Chun-tao Lai ( [email protected] ) Beijing Tongren Hospital, Capital Medical University https://orcid.org/0000-0002-4407-498X Research Keywords: Myelin oligodendrocyte glycoprotein (MOG), optic perineuritis, optic neuritis, magnetic resonance imaging, clinical features,epilepsy,meningitis,meningoencephalitis,prognosis Posted Date: March 16th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-266843/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/16 Abstract Objective To shed light on the clinical characteristics, magnet resonance imaging(MRI) changes, and prognosis of myelin oligodendrocyte glycoprotein antibody (MOG-IgG) positive OPN in Han Chinese. Methods We observed 39 MOG-IgG positive patients in our ward from January 1, 2017 , to December 31, 2019. Twenty patients met OPN inclusion criteria included contrast enhancement surrounding the optic nerve, and at least one of the following clinical symptoms: 1) reduction of visual acuity, 2) impairment of visual eld, and 3) eye pain. Single course group(n=11) and recurrence group(n=9) were used for comparison. Outcome variables included Wingerchuk visual acuity classication. Results Of the 20 patients with MOG-IgG positive OPN, 12(60%) were women. Ten cases (50%) presented with bilateral and 17 eyes (56.67%) with severe visual loss (SVL,≤ 0.1). -

Regulation of Myelin Structure and Conduction Velocity by Perinodal Astrocytes
Correction NEUROSCIENCE Correction for “Regulation of myelin structure and conduc- tion velocity by perinodal astrocytes,” by Dipankar J. Dutta, Dong Ho Woo, Philip R. Lee, Sinisa Pajevic, Olena Bukalo, William C. Huffman, Hiroaki Wake, Peter J. Basser, Shahriar SheikhBahaei, Vanja Lazarevic, Jeffrey C. Smith, and R. Douglas Fields, which was first published October 29, 2018; 10.1073/ pnas.1811013115 (Proc. Natl. Acad. Sci. U.S.A. 115,11832–11837). The authors note that the following statement should be added to the Acknowledgments: “We acknowledge Dr. Hae Ung Lee for preliminary experiments that informed the ultimate experimental approach.” Published under the PNAS license. Published online June 10, 2019. www.pnas.org/cgi/doi/10.1073/pnas.1908361116 12574 | PNAS | June 18, 2019 | vol. 116 | no. 25 www.pnas.org Downloaded by guest on October 2, 2021 Regulation of myelin structure and conduction velocity by perinodal astrocytes Dipankar J. Duttaa,b, Dong Ho Wooa, Philip R. Leea, Sinisa Pajevicc, Olena Bukaloa, William C. Huffmana, Hiroaki Wakea, Peter J. Basserd, Shahriar SheikhBahaeie, Vanja Lazarevicf, Jeffrey C. Smithe, and R. Douglas Fieldsa,1 aSection on Nervous System Development and Plasticity, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892; bThe Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817; cMathematical and Statistical Computing Laboratory, Office of Intramural Research, Center for Information -
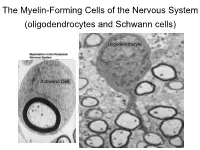
The Myelin-Forming Cells of the Nervous System (Oligodendrocytes and Schwann Cells)
The Myelin-Forming Cells of the Nervous System (oligodendrocytes and Schwann cells) Oligodendrocyte Schwann Cell Oligodendrocyte function Saltatory (jumping) nerve conduction Oligodendroglia PMD PMD Saltatory (jumping) nerve conduction Investigating the Myelinogenic Potential of Individual Oligodendrocytes In Vivo Sparse Labeling of Oligodendrocytes CNPase-GFP Variegated expression under the MBP-enhancer Cerebral Cortex Corpus Callosum Cerebral Cortex Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Ant Commissure Corpus Callosum Cerebral Cortex Caudate Putamen Piriform Cortex Corpus Callosum Ant Commissure Characterization of Oligodendrocyte Morphology Cerebral Cortex Corpus Callosum Caudate Putamen Cerebellum Brain Stem Spinal Cord Oligodendrocytes in disease: Cerebral Palsy ! CP major cause of chronic neurological morbidity and mortality in children ! CP incidence now about 3/1000 live births compared to 1/1000 in 1980 when we started intervening for ELBW ! Of all ELBW {gestation 6 mo, Wt. 0.5kg} , 10-15% develop CP ! Prematurely born children prone to white matter injury {WMI}, principle reason for the increase in incidence of CP ! ! 12 Cerebral Palsy Spectrum of white matter injury ! ! Macro Cystic Micro Cystic Gliotic Khwaja and Volpe 2009 13 Rationale for Repair/Remyelination in Multiple Sclerosis Oligodendrocyte specification oligodendrocytes specified from the pMN after MNs - a ventral source of oligodendrocytes -

Targeting Myelin Lipid Metabolism As a Potential Therapeutic Strategy in a Model of CMT1A Neuropathy
ARTICLE DOI: 10.1038/s41467-018-05420-0 OPEN Targeting myelin lipid metabolism as a potential therapeutic strategy in a model of CMT1A neuropathy R. Fledrich 1,2,3, T. Abdelaal 1,4,5, L. Rasch1,4, V. Bansal6, V. Schütza1,3, B. Brügger7, C. Lüchtenborg7, T. Prukop1,4,8, J. Stenzel1,4, R.U. Rahman6, D. Hermes 1,4, D. Ewers 1,4, W. Möbius 1,9, T. Ruhwedel1, I. Katona 10, J. Weis10, D. Klein11, R. Martini11, W. Brück12, W.C. Müller3, S. Bonn 6,13, I. Bechmann2, K.A. Nave1, R.M. Stassart 1,3,12 & M.W. Sereda1,4 1234567890():,; In patients with Charcot–Marie–Tooth disease 1A (CMT1A), peripheral nerves display aberrant myelination during postnatal development, followed by slowly progressive demye- lination and axonal loss during adult life. Here, we show that myelinating Schwann cells in a rat model of CMT1A exhibit a developmental defect that includes reduced transcription of genes required for myelin lipid biosynthesis. Consequently, lipid incorporation into myelin is reduced, leading to an overall distorted stoichiometry of myelin proteins and lipids with ultrastructural changes of the myelin sheath. Substitution of phosphatidylcholine and phosphatidylethanolamine in the diet is sufficient to overcome the myelination deficit of affected Schwann cells in vivo. This treatment rescues the number of myelinated axons in the peripheral nerves of the CMT rats and leads to a marked amelioration of neuropathic symptoms. We propose that lipid supplementation is an easily translatable potential therapeutic approach in CMT1A and possibly other dysmyelinating neuropathies. 1 Department of Neurogenetics, Max-Planck-Institute of Experimental Medicine, Göttingen 37075, Germany. -

Full Disclosures
ARTICLE OPEN ACCESS Differential Binding of Autoantibodies to MOG Isoforms in Inflammatory Demyelinating Diseases Kathrin Schanda, MSc, Patrick Peschl, PhD, Magdalena Lerch, MSc, Barbara Seebacher, PhD, Correspondence Swantje Mindorf, MSc, Nora Ritter, MSc, Monika Probst, PhD, Harald Hegen, MD, PhD, Dr. Reindl [email protected] Franziska Di Pauli, MD, PhD, Eva-Maria Wendel, MD, Christian Lechner, MD, Matthias Baumann, MD, Sara Mariotto, MD, PhD, Sergio Ferrari, MD, Albert Saiz, MD, PhD, Michael Farrell, MD, Maria Isabel S. Leite, MD, DPhil, Sarosh R. Irani, MD, DPhil, Jacqueline Palace, FRCP, DM, Andreas Lutterotti, MD, Tania Kumpfel,¨ MD, Sandra Vukusic, MD, Romain Marignier, MD, PhD, Patrick Waters, PhD, Kevin Rostasy, MD, Thomas Berger, MD, Christian Probst, PhD, Romana Hoftberger,¨ MD, and Markus Reindl, PhD Neurol Neuroimmunol Neuroinflamm 2021;8:e1027. doi:10.1212/NXI.0000000000001027 Abstract Objective To analyze serum immunoglobulin G (IgG) antibodies to major isoforms of myelin oligo- dendrocyte glycoprotein (MOG-alpha 1-3 and beta 1-3) in patients with inflammatory de- myelinating diseases. Methods Retrospective case-control study using 378 serum samples from patients with multiple sclerosis (MS), patients with non-MS demyelinating disease, and healthy controls with MOG alpha-1- IgG positive (n = 202) or negative serostatus (n = 176). Samples were analyzed for their reactivity to human, mouse, and rat MOG isoforms with and without mutations in the extra- cellular MOG Ig domain (MOG-ecIgD), soluble MOG-ecIgD, and myelin from multiple species using live cell-based, tissue immunofluorescence assays and ELISA. Results The strongest IgG reactivities were directed against the longest MOG isoforms alpha-1 (the currently used standard test for MOG-IgG) and beta-1, whereas the other isoforms were less frequently recognized. -

The Effects of Normal Aging on Myelin and Nerve Fibers: a Review∗
Journal of Neurocytology 31, 581–593 (2002) The effects of normal aging on myelin and nerve fibers: A review∗ ALAN PETERS Department of Anatomy and Neurobiology, Boston University School of Medicine, 715, Albany Street, Boston, MA 02118, USA [email protected] Received 7 January 2003; revised 4 March 2003; accepted 5 March 2003 Abstract It was believed that the cause of the cognitive decline exhibited by human and non-human primates during normal aging was a loss of cortical neurons. It is now known that significant numbers of cortical neurons are not lost and other bases for the cognitive decline have been sought. One contributing factor may be changes in nerve fibers. With age some myelin sheaths exhibit degenerative changes, such as the formation of splits containing electron dense cytoplasm, and the formation on myelin balloons. It is suggested that such degenerative changes lead to cognitive decline because they cause changes in conduction velocity, resulting in a disruption of the normal timing in neuronal circuits. Yet as degeneration occurs, other changes, such as the formation of redundant myelin and increasing thickness suggest of sheaths, suggest some myelin formation is continuing during aging. Another indication of this is that oligodendrocytes increase in number with age. In addition to the myelin changes, stereological studies have shown a loss of nerve fibers from the white matter of the cerebral hemispheres of humans, while other studies have shown a loss of nerve fibers from the optic nerves and anterior commissure in monkeys. It is likely that such nerve fiber loss also contributes to cognitive decline, because of the consequent decrease in connections between neurons. -

Schwann Cells, but Not Oligodendrocytes, Depend Strictly
RESEARCH ARTICLE Schwann cells, but not Oligodendrocytes, Depend Strictly on Dynamin 2 Function Daniel Gerber1†, Monica Ghidinelli1†, Elisa Tinelli1, Christian Somandin1, Joanne Gerber1, Jorge A Pereira1, Andrea Ommer1, Gianluca Figlia1, Michaela Miehe1, Lukas G Na¨ geli1, Vanessa Suter1, Valentina Tadini1, Pa´ ris NM Sidiropoulos1, Carsten Wessig2, Klaus V Toyka2, Ueli Suter1* 1Department of Biology, Institute of Molecular Health Sciences, Swiss Federal Institute of Technology, ETH Zurich, Zurich, Switzerland; 2Department of Neurology, University Hospital of Wu¨ rzburg, University of Wu¨ rzburg, Wu¨ rzburg, Germany Abstract Myelination requires extensive plasma membrane rearrangements, implying that molecules controlling membrane dynamics play prominent roles. The large GTPase dynamin 2 (DNM2) is a well-known regulator of membrane remodeling, membrane fission, and vesicular trafficking. Here, we genetically ablated Dnm2 in Schwann cells (SCs) and in oligodendrocytes of mice. Dnm2 deletion in developing SCs resulted in severely impaired axonal sorting and myelination onset. Induced Dnm2 deletion in adult SCs caused a rapidly-developing peripheral neuropathy with abundant demyelination. In both experimental settings, mutant SCs underwent prominent cell death, at least partially due to cytokinesis failure. Strikingly, when Dnm2 was deleted in adult SCs, non-recombined SCs still expressing DNM2 were able to remyelinate fast and efficiently, accompanied by neuropathy remission. These findings reveal a remarkable self-healing capability of peripheral nerves that are affected by SC loss. In the central nervous system, however, *For correspondence: we found no major defects upon Dnm2 deletion in oligodendrocytes. [email protected] DOI: https://doi.org/10.7554/eLife.42404.001 †These authors contributed equally to this work Competing interests: The Introduction authors declare that no Motor, sensory, and cognitive functions of the nervous system require rapid and refined impulse competing interests exist.