Water Requirements of the Pulp and Paper Industry, by O
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardboard and Brown Paper Bags Office Paper, Newspaper, Junk Mail, Magazines, and Catalogs
Recycling Center 801 Diamond Valley Drive Open: Daily to the public during daylight hours This guide will help you properly prepare your recyclable materials for drop-off at the Town of Windsor Recycle Center. This is a drop-off facility. It does not have a buy-back option and is for use by residents and small businesses. Following this information will help maintain the facility and the recycling program for the benefit of the community. IMPORTANT… • Do not leave your recyclables in plastic bags. Plastic bags are NOT recyclable! • The plastic item must be a BOTTLE or JAR. with a #1 or #2 on the bottom. • 99 percent of these will have a screw-on plastic lid (which isn’t recyclable). • Plastic containers with a #3 - #7 on the bottom are NOT acceptable. • Tubs, buckets, deli plates, microwave/fast food trays, wrappers, Styrofoam, toys, patio furniture, etc. are NOT acceptable. • Plastic bottles larger than 2.5 gallons are NOT acceptable. • Syringes and other medical supplies are NOT acceptable. Cardboard and Brown Paper Bags Corrugated cardboard is easy to recognize. It is made of paper and has an arched layer called “fluting” between smooth sheets called “liners”. The drop-off site has two 40-yard hydraulic compactor units for collecting corrugated cardboard and brown paper bags. The compaction system is self-activated by depositing the prepared materials into a six-inch tall slot. Flatten boxes. Cut or tear large boxes into sections no larger than 4 feet by 4 feet to prevent jamming the machine. No wet, waxed-coated or food-contaminated boxes. -

The EMA Guide to Envelopes and Mailing
The EMA Guide to Envelopes & Mailing 1 Table of Contents I. History of the Envelope An Overview of Envelope Beginnings II. Introduction to the Envelope Envelope Construction and Types III. Standard Sizes and How They Originated The Beginning of Size Standardization IV. Envelope Construction, Seams and Flaps 1. Seam Construction 2. Glues and Flaps V. Selecting the Right Materials 1. Paper & Other Substrates 2. Window Film 3. Gums/Adhesives 4. Inks 5. Envelope Storage 6. Envelope Materials and the Environment 7. The Paper Industry and the Environment VI. Talking with an Envelope Manufacturer How to Get the Best Finished Product VII. Working with the Postal Service Finding the Information You Need VIII. Final Thoughts IX. Glossary of Terms 2 Forward – The EMA Guide to Envelopes & Mailing The envelope is only a folded piece of paper yet it is an important part of our national communications system. The power of the envelope is the power to touch someone else in a very personal way. The envelope has been used to convey important messages of national interest or just to say “hello.” It may contain a greeting card sent to a friend or relative, a bill or other important notice. The envelope never bothers you during the dinner hour nor does it shout at you in the middle of a television program. The envelope is a silent messenger – a very personal way to tell someone you care or get them interested in your product or service. Many people purchase envelopes over the counter and have never stopped to think about everything that goes into the production of an envelope. -

Triangle Accordion Books Part 2
“Part Two: Assembling the book” Time from start to finish = 1 hour You will need the following materials: • Heavy paper, which will be cut into three 4 5/8” X 20” strips, the best option is to cut these strips from one large 22” X 30” sheet of watercolor paper (this will be used for the inner “accordion” of the book) • 2 pieces of chipboard or thick cardboard, 6x6 inches each • Painted paper or scrapbooking paper at least 6x6 inches in size • Pencil • Ruler • Glue (regular white glue or glue sticks) • Clean scrap paper, computer paper or newsprint is good • X-acto Knife or scissors • Cutting matt (or a piece of scrap cardboard to protect your work surface) Brief description: The next part of our process is to measure, cut and assemble your book. This part is made up of several smaller steps within each major step and requires precise measurement and patience. For Part Two, I suggest making a cup of your favorite tea and putting on some relaxing music in the background. While, this may be the most challenging step for some, if you follow the instructions closely and don’t rush, you should come out of it with a fully assembled book ready for creative alteration and decoration! For those new to bookmaking here are a few helpful hints: • Measure! Measure! Measure!: Nothing is more disappointing than assembling several pieces only to find at the final step that they don’t quite fit, especially when you’re out of materials to try again! During this process, we will be measuring out strips of paper, then folding, cutting and gluing them together at specific points. -

Mountain Pine Beetle-Attacked Lodgepole Pine for Pulp and Papermaking
Operational extractives management from- mountain pine beetle-attacked lodgepole pine for pulp and papermaking Larry Allen and Vic Uloth Mountain Pine Beetle Working Paper 2007-15 Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre, 506 West Burnside Road, Victoria, BC V8Z 1M5 (250) 363-0600 • cfs.nrcan.gc.ca/regions/pfc Natural Resources Ressources naturelles Canada Canada Canadian Forest Service canadien Service des forêts Operational extractives management from mountain pine beetle-attacked lodgepole pine for pulp and papermaking Larry Allen and Vic Uloth Mountain Pine Beetle Initiative W orking Paper 2007œ15 Paprican 3800 W esbrook Mall Vancouver, B.C. V6S 2L9 Mountain Pine Beetle Initiative PO # 8.43 Natural Resources Canada Canadian Forest Service Pacific Forestry Centre 506 W est Burnside Road Victoria, British Columbia V8Z 1M5 Canada 2007 ≤ Her Majesty the Queen in Right of Canada 2007 Printed in Canada Library and Archives Canada Cataloguing in Publication Allen, Larry Operational extractives m anagem ent from m ountain pine beetle-attached lodgepole pine from pulp and paperm aking / Larry Allen and Vic Uloth. (Mountain Pine Beetle Initiative working paper 2007-15) "Mountain Pine Beetle Initiative, Canadian Forest Service". "MPBI Project # 8.43". "Paprican". Includes bibliographical references: p. Includes abstract in French. ISBN 978-0-662-46480-8 Cat. no.: Fo143-3/2007-15E 1. Pulping--British Colum bia--Quality control. 2. Pulping--Alberta--Quality control. 3. Paper m ills-- Econom ic aspects--British Colum bia. 4. Pulp m ills--Econom ic aspects--Alberta. 5. Lodgepole pine--Diseases and pests–Econom ic aspects. 6. Mountain pine beetle--Econom ic aspects. -

Basics of Kraft Pulping
Lignin Wood is composed of many chemical components, primarily extractives, carbohydrates, and lignin, which are distributed nonuniformly as the result of anatomical structure. Lignin is derived from the Latin term lignum, which means wood.1 Anselme Payen (1838) was the first to recognize the composite nature of wood and referred to a carbon- rich substance as the “encrusting material” which embedded cellulose in the wood. Schulze (1865) later defined this encrusting material as lignin. Lignin has been described as a random, three-dimensional network polymer comprised of variously linked phenylpropane units.2 Lignin is the second most abundant biological material on the planet, exceeded only by cellulose and hemicellulose, and comprises 15-25% of the dry weight of woody plants. This macromolecule plays a vital role in providing mechanical support to bind plant fibers together. Lignin also decreases the permeation of water through the cell walls of the xylem, thereby playing an intricate role in the transport of water and nutrients. Finally, lignin plays an important function in a plant’s natural defense against degradation by impeding penetration of destructive enzymes through the cell wall. Although lignin is necessary to trees, it is undesirable in most chemical papermaking fibers and is removed by pulping and bleaching processes. 1.1.1 Biosynthesis Plant lignins can be broadly divided into three classes: softwood (gymnosperm), hardwood (angiosperm) and grass or annual plant (graminaceous) lignin.3 Three different phenylpropane units, or monolignols, are responsible for lignin biosynthesis.4 Guaiacyl lignin is composed principally of coniferyl alcohol units, while guaiacyl-syringyl lignin contains monomeric units from coniferyl and sinapyl alcohol. -

Corrugated Board Structure: a Review M.C
ISSN: 2395-3594 IJAET International Journal of Application of Engineering and Technology Vol-2 No.-3 Corrugated Board Structure: A Review M.C. Kaushal1, V.K.Sirohiya2 and R.K.Rathore3 1 2 Assistant Prof. Mechanical Engineering Department, Gwalior Institute of Information Technology,Gwalior, Assistant Prof. Mechanical Engineering 3 Departments, Gwalior Engineering College, Gwalior, M. Tech students Maharanapratap College of Technology, Gwalior, [email protected] [email protected] [email protected] ABSTRACT Corrugated board is widely used in the packing industry. The main advantages are lightness, recyclability and low cost. This makes the material the best choice to produce containers devoted to the shipping of goods. Furthermore examples of structure design based on corrugated boards can be found in different fields. Structural analysis of paperboard components is a crucial topic in the design of containers. It is required to investigate their strength properties because they have to protect the goods contained from lateral crushing and compression loads due to stacking. However in this paper complete and detailed information are presented. Keywords: - corrugated boards, recyclability, compression loads. Smaller flutes offer printability advantages as well as I. INTRODUCTION structural advantages for retail packaging. Corrugated board is essentially a paper sandwich consisting of corrugated medium layered between inside II. HISTORY and outside linerboard. On the production side, corrugated In 1856 the first known corrugated material was patented is a sub-category of the paperboard industry, which is a for sweatband lining in top hats. During the following four sub-category of the paper industry, which is a sub-category decades other forms of corrugated material were used as of the forest products industry. -
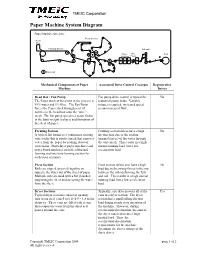
Paper Machine System Diagram
TMEIC Corporation Paper Machine System Diagram Paper Machine One-Line Press Section Head Box Forming Section Calender Dryer Size Reel Sections Press Driven roll Mechanical Components of Paper Associated Drive Control Concepts Regenerative Machine Drives Head Box / Fan Pump Fan pump drive control is typical for No The Paper stock at this point in the process is industrial pump loads. Variable 99% water and 1% fiber. The Fan Pump torque is required, increased speed forces the Paper stock through a set of means increased flow. nozzles in the head box onto the “wire” mesh. The fan pump speed is a major factor in the basis weight (caliper) and formation of the sheet of paper. Forming Section Forming section drives have a high No A typical flat former is a continuous rotating friction load due to the suction wire (today this is plastic) mesh that removes (normal forces) of the water through water from the paper by sucking it out of the wire mesh. This results in a high suspension. Multi-layer paper machines and normal running load, but a low paper board machines include additional acceleration load. forming sections (one forming section for each layer of paper). Press Section Press section drives also have a high No Rolls are nipped (pressed) together to load due to the strong forces in the nip squeeze the water out of the sheet of paper. between the rolls deforming the felts Multiple rolls are used with a felt (blanket) and roll. This results in a high normal supporting the sheet and accepting the water running load, but a low acceleration from the sheet. -

Final Paper Products Recovered Materials Advisory Notice Response to Public Comments
FINAL PAPER PRODUCTS RECOVERED MATERIALS ADVISORY NOTICE Response to Public Comments Office of Solid Waste U.S. Environmental Protection Agency April 1996 CONTENTS I. Introduction . 1 A. The Draft Paper Products Recovered Materials Advisory Notice . 1 B. Overview of the RCRA Requirements . 1 C. Overview of the Executive Order Provisions for Paper . 3 II. Applicability . 5 A. Background. 5 B. Comments and Agency Response. 5 III. EPA's Objectives . 7 A. Background. 7 B. Comments and Agency Response. 7 IV. EPA's Approach to Recommendations. 11 A. Background . 11 B. Comments and Agency Response . 11 V. EPA's Methodology . 14 A. Background . 14 B. Comments and Agency Response . 14 VI. Recommendations for Printing and Writing Papers. 15 A. Background . 15 B. Comments and Agency Response . 19 VII. Recommendations for Newsprint . 27 A. Background . 27 B. Comments and Agency Response . 27 VIII. Recommendations for Tissue Products. 31 A. Background . 31 B. Comments and Agency Response . 32 IX. Recommendations for Paperboard and Packaging Products . 36 A. Background . 36 B. Comments and Agency Response . 38 X. Recommendations for Miscellaneous Paper Products. 45 A. Background . 45 B. Comments and Agency Response . 45 XI. Recommendations for Measurement. 46 A. Background . 46 B. Comments and Agency Response . 46 XII. Recommendations for Specifications. 48 A. Background . 48 B. Comments and Agency Response . 48 XIII. Recommendations for Recyclability. 49 A. Background . 49 B. Comments and Agency Response . 49 XIV. Definitions . 51 A. Background . 51 B. Comments and Agency Response . 51 XV. Certification and Verification . 54 A. Background . 55 B. Comments and Agency Response . 55 XVI. Sawdust as Recovered Fiber. -

Articles of Paper Pulp, of Paper Or of Paperboard
Chapter 48 Paper and paperboard; articles of paper pulp, of paper or of paperboard Notes. 1.- For the purposes of this Chapter, except where the context otherwise requires, a reference to “paper” includes references to paperboard (irrespective of thickness or weight per m²). 2.- This Chapter does not cover : (a) Articles of Chapter 30; (b) Stamping foils of heading 32.12; (c) Perfumed papers or papers impregnated or coated with cosmetics (Chapter 33); (d) Paper or cellulose wadding impregnated, coated or covered with soap or detergent (heading 34.01), or with polishes, creams or similar preparations (heading 34.05); (e) Sensitised paper or paperboard of headings 37.01 to 37.04; (f) Paper impregnated with diagnostic or laboratory reagents (heading 38.22); (g) Paper-reinforced stratified sheeting of plastics, or one layer of paper or paperboard coated or covered with a layer of plastics, the latter constituting more than half the total thickness, or articles of such materials, other than wall coverings of heading 48.14 (Chapter 39); (h) Articles of heading 42.02 (for example, travel goods); (ij) Articles of Chapter 46 (manufactures of plaiting material); (k) Paper yarn or textile articles of paper yarn (Section XI); (l) Articles of Chapter 64 or Chapter 65; (m) Abrasive paper or paperboard (heading 68.05) or paper- or paperboard-backed mica (heading 68.14) (paper and paperboard coated with mica powder are, however, to be classified in this Chapter); (n) Metal foil backed with paper or paperboard (generally Section XIV or XV); (o) Articles of heading 92.09; (p) Articles of Chapter 95 (for example, toys, games, sports requisites); or (q) Articles of Chapter 96 (for example, buttons, sanitary towels (pads) and tampons, napkins (diapers) and napkin liners for babies). -

THE REVISION of EU ECOLABEL CRITERIA for Converted Paper Products
THE REVISION OF EU ECOLABEL CRITERIA for Converted Paper Products Draft Preliminary Report Malgorzata Kowalska, Antonios Konstantas, Oliver Wolf Marzia Traverso, Rose Nangah Mankaa, Sabrina Neugebauer November 2018 EUR xxxxx xx 1 This publication is a Science for Policy report by the Joint Research Centre, the European Commission’s in-house science service. It aims to provide evidence-based scientific support to the European policy-making process. The scientific output expressed does not imply a policy position of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of this publication. Contact information Name: Address: E-mail: Tel.: JRC Science Hub https://ec.europa.eu/jrc JRCxxxxx EUR xxxxx xx PDF ISBN xxx-xx-xx-xxxxx-x ISSN xxxx-xxxx doi:xx.xxxx/xxxxxx XX-NA-xxxxx-EN-N Print ISBN xxx-xx-xx-xxxxx-x ISSN xxxx-xxxx doi:xx.xxxxx/xxxxxx XX-NA-xxxxx-EN-C © European Union, 20xx Reproduction is authorised provided the source is acknowledged. How to cite: Authors; title; EUR; doi All images © European Union 20xx, except: 2 Table of contents ABSTRACT ............................................................................................................ 3 Executive summary ............................................................................................... 3 1. Introduction ...................................................................................................... 4 2. Task 1: Scope and definition analysis .................................................................. -

Paper Grades
720 PAPERMAKING / Paper Grades Further Reading Biermann CJ (1996) Handbook of Pulping and Papermak- ing. San Diego: Academic Press. Borch J, Lyne MB, Mark RE, and Habeger Jr, CC (eds) (2002) Handbook of Physical Testing of Paper, vol. 2, 2nd edn. New York: Marcel Dekker. Britt KW (1975) Handbook of Pulp and Paper Technology. New York: Van Nostrand Reinhold. Clark Jd’A (1985) Pulp Technology and Treatment for Paper, 2nd edn. San Francisco: Miller Freeman. Dieson M (1998) Papermaking Science and Technology. Atlanta, GA: Tappi Press. Gavelin G (1998) Paper Machine Design and Operation. Vancouver, BC: Angus Wilde. Hunter D (1978) Papermaking: The History and Techni- que of an Ancient Craft. New York: Dover Publications. Karlsson M (2000) Papermaking, Part 2, Drying. Atlanta, GA: Tappi Press. Kline JE (1991) Paper and Paperboard: Manufacturing and Converting Fundamentals. San Francisco: Miller Freeman. Kojio M (1999) Papermaking: Finishing. Atlanta, GA: Tappi Press. Levlin J-E and So¨ derhelm L (1999) Pulp and Paper Testing. Atlanta, GA: Tappi Press. Mark RE, Habeger Jr, CC, Borch J, and Lyne MB (eds) (2002) Handbook of Physical Testing of Paper, vol. 1, 2nd edn. New York: Marcel Dekker. Patrick KL (1999) Primer of Pulping and Paper Making: Technologies and Production Practices. San Francisco, CA: Miller Freeman Books. Figure 20 Calender of the pilot papermachine at SUNY Paulapuro H (2000) Papermaking Part 1: Stock Prepara- College of Environmental Science and Forestry. The sheet tion and Wet End. Atlanta, GA: Tappi Press. passes through multiple nips to smooth the surface of the sheet. Savolainen A (1998) Paper and Paperboard Converting: Papermaking Science and Technology. -

A3 Paper Size: ISO Paper Size 297 X 420Mm Used for Small Poster Or Small News Paper
A3 Paper size: ISO paper size 297 x 420mm used for small poster or small news paper. A4 Paper size: ISO paper size 210 x 297mm used for Letterhead. Accordion fold: Bindery term, two or more parallel folds which open like an accordion. Acetate: a transparent sheet placed over artwork allowing the artist to write instructions or indicate where second colour is to be placed. Acid Resist: An acid-proof protective coating applied to metal plates prior to etching. Against the grain: At right angles to direction of paper grain. Alteration: Change in copy of specifications after production has begun. Aqueous Coating: Coating in a water base and applied like ink by a printing press to protect and enhance the printing underneath. Artboard: Alternate term for mechanical art. Ascender: any part of a lower case letter extending above the x-height. For example, the upper half of the vertical in the letters b or h. Authors corrections: changes made to the copy by the author after typesetting but not including those made as a result of errors in keying in the copy. Back up: Printing the second side of a sheet already printed on one side. Backslant: Letters that slant the opposite way from italic characters. Banding: Method of packaging printed pieces of paper using rubber or paper bands. Basis weight: Weight in pounds of a ream of paper cut to the basic size for its grade. Bind: To fasten sheets or signatures with wire, thread, glue. or by other means. Bindery: The finishing department of a print shop or firm specializing in finishing printed products.