Mesh Terms for 2002
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acidic Phospholipids,47, 58 Acidification Steps, 23 Adsorbent, 53
Index acidic phospholipids,47, 58 ATPase, 22, 107 acidification steps, 23 auto-oxidation, 48,49 adsorbent, 53 aequorin, 95 aggregated mitochondria, 23 bacterial cells, 10 alcohols, 49 bacteriorhodopsin (BR), 218,232 alkaline hydrolysis, I bee venom, 121 alkyl esters, 57, 59 binders,53 amide bond, 129 silica gel, 53, 65, 176 amide linkage, 14 silica gel H, 176 amino alcohol, II bioactive phospholipids, 144 amino-group labelling reagents, 121 blanching, 50 aminopeptidase, 17 buoyant density, 16 aminophospholipid, 113, 118 butyl hydroxy toluene (BHT), 49,212 aminophospholipid pump, 119, 140 aminophospholipid translocase, 11 3 amphotericin B (AmB), 107 Cal+ -ATPases, 28, 107 N-(1-deoxyD-fructos-1-yl) AmB, 107 Cal+ -uptake, 27 anchored lipids, 38 campesterol, 104 angular amplitude, 89 calciferol, 78 anilino-8-naphthalene sulfonate (ANS), Candida albicans, 59, 60, 61, 76, 78, 102, 94, 103 104, 108 animal cells, 10 caproic acid, 129, 130 animal tissues, 9 carotenoids, 37 anion transporter, 135 cell debris, 33 anisotropy, 81 , 89, 95, 96, 97, 102, 104, cell disintegrator, 19 105 ceramide, 14, 129, 194 antagonists, 178 ceramide monohexosides ( CMH), 60 antioxidant, 49, 50 cerebroside, 14 apolar lipids, 56, 57, 69 chemical probes,112 arachidonic acid (AA), 144,153, 161 chloroplast, 32, 33 artificial membrane, 83 isolation, 32, 33 ascending chromatography, 54 purification, 33 asymmetric topology, 128 cholesterol, 14, 15,212 asymmetry, 112, 113,119 choline, 7, 9 atebrin, 95 chromatograms, 55 Index 249 chromatographic analyses, 52 ELISA, 146, 148 -

Pfeiffer Syndrome Type II Discovered Perinatally
Diagnostic and Interventional Imaging (2012) 93, 785—789 CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector LETTER / Musculoskeletal imaging Pfeiffer syndrome type II discovered perinatally: Report of an observation and review of the literature a,∗ a a a H. Ben Hamouda , Y. Tlili , S. Ghanmi , H. Soua , b c b a S. Jerbi , M.M. Souissi , H. Hamza , M.T. Sfar a Unité de néonatologie, service de pédiatrie, CHU Tahar Sfar, 5111 Mahdia, Tunisia b Service de radiologie, CHU Tahar Sfar, 5111 Mahdia, Tunisia c Service de gynéco-obstétrique, CHU Tahar Sfar, 5111 Mahdia, Tunisia Pfeiffer syndrome, described for the first time by Pfeiffer in 1964, is a rare hereditary KEYWORDS condition combining osteochondrodysplasia with craniosynostosis [1]. This syndrome is Pfeiffer syndrome; also called acrocephalosyndactyly type 5, which is divided into three sub-types. Type I Cloverleaf skull; is the classic Pfeiffer syndrome, with autosomal dominant transmission, often associated Craniosynostosis; with normal intelligence. Types II and III occur as sporadic cases in individuals who have Syndactyly; craniosynostosis with broad thumbs, broad big toes, ankylosis of the elbows and visceral Prenatal diagnosis abnormalities [2]. We report a case of Pfeiffer syndrome type II, discovered perinatally, which is distinguished from type III by the skull appearing like a cloverleaf, and we shall discuss the clinical, radiological and evolutive features and the advantage of prenatal diagnosis of this syndrome with a review of the literature. Observation The case involved a male premature baby born at 36 weeks of amenorrhoea with multiple deformities at birth. The parents were not blood-related and in good health who had two other boys and a girl with normal morphology. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Contig Protein Description Symbol Anterior Posterior Ratio
Table S2. List of proteins detected in anterior and posterior intestine pooled samples. Data on protein expression are mean ± SEM of 4 pools fed the experimental diets. The number of the contig in the Sea Bream Database (http://nutrigroup-iats.org/seabreamdb) is indicated. Contig Protein Description Symbol Anterior Posterior Ratio Ant/Pos C2_6629 1,4-alpha-glucan-branching enzyme GBE1 0.88±0.1 0.91±0.03 0.98 C2_4764 116 kDa U5 small nuclear ribonucleoprotein component EFTUD2 0.74±0.09 0.71±0.05 1.03 C2_299 14-3-3 protein beta/alpha-1 YWHAB 1.45±0.23 2.18±0.09 0.67 C2_268 14-3-3 protein epsilon YWHAE 1.28±0.2 2.01±0.13 0.63 C2_2474 14-3-3 protein gamma-1 YWHAG 1.8±0.41 2.72±0.09 0.66 C2_1017 14-3-3 protein zeta YWHAZ 1.33±0.14 4.41±0.38 0.30 C2_34474 14-3-3-like protein 2 YWHAQ 1.3±0.11 1.85±0.13 0.70 C2_4902 17-beta-hydroxysteroid dehydrogenase 14 HSD17B14 0.93±0.05 2.33±0.09 0.40 C2_3100 1-acylglycerol-3-phosphate O-acyltransferase ABHD5 ABHD5 0.85±0.07 0.78±0.13 1.10 C2_15440 1-phosphatidylinositol phosphodiesterase PLCD1 0.65±0.12 0.4±0.06 1.65 C2_12986 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase delta-1 PLCD1 0.76±0.08 1.15±0.16 0.66 C2_4412 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase gamma-2 PLCG2 1.13±0.08 2.08±0.27 0.54 C2_3170 2,4-dienoyl-CoA reductase, mitochondrial DECR1 1.16±0.1 0.83±0.03 1.39 C2_1520 26S protease regulatory subunit 10B PSMC6 1.37±0.21 1.43±0.04 0.96 C2_4264 26S protease regulatory subunit 4 PSMC1 1.2±0.2 1.78±0.08 0.68 C2_1666 26S protease regulatory subunit 6A PSMC3 1.44±0.24 1.61±0.08 -

Bioluminescent Properties of Semi-Synthetic Obelin and Aequorin Activated by Coelenterazine Analogues with Modifications of C-2, C-6, and C-8 Substituents
International Journal of Molecular Sciences Article Bioluminescent Properties of Semi-Synthetic Obelin and Aequorin Activated by Coelenterazine Analogues with Modifications of C-2, C-6, and C-8 Substituents 1, 2,3, 1 2, Elena V. Eremeeva y , Tianyu Jiang y , Natalia P. Malikova , Minyong Li * and Eugene S. Vysotski 1,* 1 Photobiology Laboratory, Institute of Biophysics SB RAS, Federal Research Center “Krasnoyarsk Science Center SB RAS”, Krasnoyarsk 660036, Russia; [email protected] (E.V.E.); [email protected] (N.P.M.) 2 Key Laboratory of Chemical Biology (MOE), Department of Medicinal Chemistry, School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China; [email protected] 3 State Key Laboratory of Microbial Technology, Shandong University–Helmholtz Institute of Biotechnology, Shandong University, Qingdao 266237, China * Correspondence: [email protected] (M.L.); [email protected] (E.S.V.); Tel.: +86-531-8838-2076 (M.L.); +7-(391)-249-44-30 (E.S.V.); Fax: +86-531-8838-2076 (M.L.); +7-(391)-290-54-90 (E.S.V.) These authors contributed equally to this work. y Received: 23 June 2020; Accepted: 27 July 2020; Published: 30 July 2020 Abstract: Ca2+-regulated photoproteins responsible for bioluminescence of a variety of marine organisms are single-chain globular proteins within the inner cavity of which the oxygenated coelenterazine, 2-hydroperoxycoelenterazine, is tightly bound. Alongside with native coelenterazine, photoproteins can also use its synthetic analogues as substrates to produce flash-type bioluminescence. However, information on the effect of modifications of various groups of coelenterazine and amino acid environment of the protein active site on the bioluminescent properties of the corresponding semi-synthetic photoproteins is fragmentary and often controversial. -

Study Sheet #1
Pathogens 1. Acinetobacter a. A. calcoaceticus i. Animals, Man – Opportunistic Infections 2. Actinobacillus a. A. actinomycetemocomitans i. Animals – Opportunistic Infection ii. Man – Opportunistic Infection b. A. equuli i. Equine – Actinobacillosis c. A. lignieresii i. Bovine – Actinobacillosis d. A. pleuropneumoniae i. Porcine – Pleuropneumonia 3. Aeromonas a. A. hydrophilia i. Animals, Man – Opportunistic Infection 4. Alcaligenes a. Alcaligenes species i. Animals, Man – Opportunistic Infection 5. Bacillus a. B. anthracis i. Animals, Man – Anthrax b. B. cereus i. Man – Food Poisoning 6. Bordetella a. B. bronchiseptica i. Canine – Tracheobronchitis (Kennel Cough) ii. Porcine – Atrophic Rhinitis b. B. pertussis i. Man – Pertussis (Whooping Cough) 7. Brucella a. B. abortus i. Bovine –Abortion, Orchitis b. B. canis i. Canine - Abortion, Orchitis c. B. melitensis i. Ovine - Abortion, Orchitis d. B. ovis i. Ovine - Abortion, Orchitis e. B. suis i. Porcine - Abortion, Orchitis 8. Burkholderia a. B. mallei i. Equine – Glanders b. B. pseudomallei 9. Campylobacter a. C. fetus fetus i. Ovine – Epizootic abortion b. C. fetus veneralis i. Bovine – Campylobacteriosis c. C. jejuni i. Man – Enteritis d. i. Animal, Man – Melioidosis 10. Clostridium a. C. botulimum i. Animals, Man – Botulism b. C. chauvoei i. Bovine – Blackleg c. C. perfringens i. Ovine – Enterotoxemia ii. Man – Gas Gangrene d. C. tetani i. Animals, Man – Tetanus 11. Corynebacterium a. C. diphtheriae i. Man – Diphtheria b. C. psuedotuberculosis i. Ovine – Caseous lymphadenitis c. C. renale i. Bovine – Contagious pyelonephritis 12. Dermatophilus a. D. congolensis i. Animals, Man – Dermatophilosis 13. Erysipelothrix a. E. rhusiopathiae i. Porcine – Erysipelas ii. Man – Erysipeloid 14. Enterococcus species a. Opportunistic pathogens of humans and domestic animals b. -

Infant Antibiotic Exposure Search EMBASE 1. Exp Antibiotic Agent/ 2
Infant Antibiotic Exposure Search EMBASE 1. exp antibiotic agent/ 2. (Acedapsone or Alamethicin or Amdinocillin or Amdinocillin Pivoxil or Amikacin or Aminosalicylic Acid or Amoxicillin or Amoxicillin-Potassium Clavulanate Combination or Amphotericin B or Ampicillin or Anisomycin or Antimycin A or Arsphenamine or Aurodox or Azithromycin or Azlocillin or Aztreonam or Bacitracin or Bacteriocins or Bambermycins or beta-Lactams or Bongkrekic Acid or Brefeldin A or Butirosin Sulfate or Calcimycin or Candicidin or Capreomycin or Carbenicillin or Carfecillin or Cefaclor or Cefadroxil or Cefamandole or Cefatrizine or Cefazolin or Cefixime or Cefmenoxime or Cefmetazole or Cefonicid or Cefoperazone or Cefotaxime or Cefotetan or Cefotiam or Cefoxitin or Cefsulodin or Ceftazidime or Ceftizoxime or Ceftriaxone or Cefuroxime or Cephacetrile or Cephalexin or Cephaloglycin or Cephaloridine or Cephalosporins or Cephalothin or Cephamycins or Cephapirin or Cephradine or Chloramphenicol or Chlortetracycline or Ciprofloxacin or Citrinin or Clarithromycin or Clavulanic Acid or Clavulanic Acids or clindamycin or Clofazimine or Cloxacillin or Colistin or Cyclacillin or Cycloserine or Dactinomycin or Dapsone or Daptomycin or Demeclocycline or Diarylquinolines or Dibekacin or Dicloxacillin or Dihydrostreptomycin Sulfate or Diketopiperazines or Distamycins or Doxycycline or Echinomycin or Edeine or Enoxacin or Enviomycin or Erythromycin or Erythromycin Estolate or Erythromycin Ethylsuccinate or Ethambutol or Ethionamide or Filipin or Floxacillin or Fluoroquinolones -
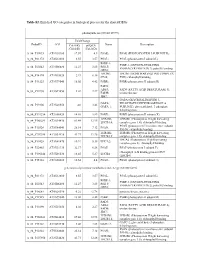
Table S2. Enriched GO Categories in Biological Process for the Shared Degs
Table S2. Enriched GO categories in biological process for the shared DEGs photosynthesis (GO ID:15979) Fold Change ProbeID AGI Col-0(R) pifQ(D) Name Description /Col-0(D) /Col-0(D) A_84_P19035 AT1G30380 17.07 4.9 PSAK; PSAK (PHOTOSYSTEM I SUBUNIT K) A_84_P21372 AT4G12800 8.55 3.57 PSAL; PSAL (photosystem I subunit L) PSBP-1; PSBP-1 (OXYGEN-EVOLVING A_84_P20343 AT1G06680 12.27 3.85 PSII-P; ENHANCER PROTEIN 2); poly(U) binding OEE2; LHCB6; LHCB6 (LIGHT HARVESTING COMPLEX A_84_P14174 AT1G15820 23.9 6.16 CP24; PSII); chlorophyll binding A_84_P11525 AT1G79040 16.02 4.42 PSBR; PSBR (photosystem II subunit R) FAD5; ADS3; FAD5 (FATTY ACID DESATURASE 5); A_84_P19290 AT3G15850 4.02 2.27 FADB; oxidoreductase JB67; GAPA (GLYCERALDEHYDE 3- GAPA; PHOSPHATE DEHYDROGENASE A A_84_P19306 AT3G26650 4.6 3.43 GAPA-1; SUBUNIT); glyceraldehyde-3-phosphate dehydrogenase A_84_P193234 AT2G06520 14.01 3.89 PSBX; PSBX (photosystem II subunit X) LHB1B1; LHB1B1 (Photosystem II light harvesting A_84_P160283 AT2G34430 89.44 32.95 LHCB1.4; complex gene 1.4); chlorophyll binding PSAN (photosystem I reaction center subunit A_84_P10324 AT5G64040 26.14 7.12 PSAN; PSI-N); calmodulin binding LHB1B2; LHB1B2 (Photosystem II light harvesting A_84_P207958 AT2G34420 41.71 12.26 LHCB1.5; complex gene 1.5); chlorophyll binding LHCA2 (Photosystem I light harvesting A_84_P19428 AT3G61470 10.91 5.36 LHCA2; complex gene 2); chlorophyll binding A_84_P22465 AT1G31330 32.37 6.58 PSAF; PSAF (photosystem I subunit F) chlorophyll A-B binding protein CP29 A_84_P190244 AT5G01530 16.45 5.27 LHCB4 -

Bacterial Skin Infections
BACTERIAL SKIN INFECTIONS SPEAKER: DR LUIZ ALBERTO BOMJARDIM PÔRTO DERMATOLOGIST BRAZIL MRSA INFECTIONS • Concept: Methicillin- resistant Staphylococcus aureus • Epidemiology: Gradual increase of resistance. • Nosocomial MRSA risk factors: Hospitalization, ICU, invasive procedures, previous antibiotic therapy, health professionals, diabetes mellitus, EV drugs, immunosuppression and chronic diseases. MRSA INFECTIONS • Community MARSA risk factors: Children, EV drugs, indigenous, homosexual men, military, prisoners and athletes. • Microorganisms more virulent by genetic characteristics. MRSA INFECTIONS • Clinic caracteristics: -Abscess, cellulitis, folliculitis, impetigo, infected wounds, external otitis, paronychia and colonization of the skin in cases of atopic dermatitis. - Increased morbidity. • Propedeutics: Culture blood, tissue or secretion. MRSA INFECTIONS • Treatment: - Pathology-specific treatment. - Prefer non-beta-lactam antibiotics, such as: clindamycin, sulfamethoxazole- trimethoprim and tetracyclines. - On suspicion of MARSA infection, start empirical antibiotics and stagger specific antibiotics by culture with antibiograma. MRSA INFECTIONS • Treatment: - Decolonization: systemic antibiotic therapy, topical 2% mupirocin, personal hygiene with antiseptic or antimicrobial solutions (iodine-povidine, chlorhexidine or triclosan). MRSA INFECTIONS • Prevention: - Avoid skin-to-skin contact and share personal belongings / clothing. - Hand washing. - Use of alcohol gels. - Cover wounds. - Isolation contact of MARSA carriers. - Early -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Alfadolone Acetate (BANM, Rinnm) 362: 1749–57
1780 General Anaesthetics blood, then to the brain; recovery is a function of the zolam, and other anaesthetics such as etomidate, propofol, ful if the patient’s cooperation is required, as conscious- removal of the anaesthetic from the brain. With inject- or ketamine. Small doses of short-acting opioids, for ex- ness soon returns once the nitrous oxide is stopped. The able anaesthetics their activity is similarly dependent ample alfentanil, fentanyl, or remifentanil, given before or neuroleptic most commonly employed was droperidol and on their ability to penetrate the blood/brain barrier and at induction allow the use of smaller induction doses of it was usually used with fentanyl although other opioids recovery in turn is governed by their redistribution and some drugs used for anaesthesia, and this technique is par- have also been used. These procedures have since evolved ticularly suitable for poor-risk patients. into conscious sedation and monitored anaesthetic care excretion. The potency of inhalational anaesthetics is After induction, muscle relaxation with a rapidly acting techniques employing newer drugs. often expressed in terms of minimum alveolar concen- depolarising neuromuscular blocker such as suxamethoni- Ketamine used alone can produce a state of dissociative trations, known as MAC values. The MAC of an um aids intubation of the patient. Longer acting, compet- anaesthesia similar to that of neuroleptanalgesia in which anaesthetic is the concentration at 1 atmosphere that itive neuromuscular blockers may then be given to allow the patient may appear to be awake but is unconscious. will produce immobility in 50% of subjects exposed to procedures such as abdominal surgery to be carried out un- Marked analgesia and amnesia are produced, but there a noxious stimulus. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known.