Infant Antibiotic Exposure Search EMBASE 1. Exp Antibiotic Agent/ 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Download
VOLUME 7 NOMOR 2 DESEMBER 2020 ISSN 2548 – 611X JURNAL BIOTEKNOLOGI & BIOSAINS INDONESIA Homepage Jurnal: http://ejurnal.bppt.go.id/index.php/JBBI IN SILICO STUDY OF CEPHALOSPORIN DERIVATIVES TO INHIBIT THE ACTIONS OF Pseudomonas aeruginosa Studi In Silico Senyawa Turunan Sefalosporin dalam Menghambat Aktivitas Bakteri Pseudomonas aeruginosa Saly Amaliacahya Aprilian*, Firdayani, Susi Kusumaningrum Pusat Teknologi Farmasi dan Medika, BPPT, Gedung LAPTIAB 610-612 Kawasan Puspiptek, Setu, Tangerang Selatan, Banten 15314 *Email: [email protected] ABSTRAK Infeksi yang diakibatkan oleh bakteri gram-negatif, seperti Pseudomonas aeruginosa telah menyebar luas di seluruh dunia. Hal ini menjadi ancaman terhadap kesehatan masyarakat karena merupakan bakteri yang multi-drug resistance dan sulit diobati. Oleh karena itu, pentingnya pengembangan agen antimikroba untuk mengobati infeksi semakin meningkat dan salah satu yang saat ini banyak dikembangkan adalah senyawa turunan sefalosporin. Penelitian ini melakukan studi mengenai interaksi tiga dimensi (3D) antara antibiotik dari senyawa turunan Sefalosporin dengan penicillin-binding proteins (PBPs) pada P. aeruginosa. Tujuan dari penelitian ini adalah untuk mengklarifikasi bahwa agen antimikroba yang berasal dari senyawa turunan sefalosporin efektif untuk menghambat aktivitas bakteri P. aeruginosa. Struktur PBPs didapatkan dari Protein Data Bank (PDB ID: 5DF9). Sketsa struktur turunan sefalosporin digambar menggunakan Marvins Sketch. Kemudian, studi mengenai interaksi antara antibiotik dan PBPs dilakukan menggunakan program Mollegro Virtual Docker 6.0. Hasil yang didapatkan yaitu nilai rerank score terendah dari kelima generasi sefalosporin, di antaranya sefalotin (-116.306), sefotetan (-133.605), sefoperazon (-160.805), sefpirom (- 144.045), dan seftarolin fosamil (-146.398). Keywords: antibiotik, penicillin-binding proteins, P. aeruginosa, sefalosporin, studi interaksi ABSTRACT Infections caused by gram-negative bacteria, such as Pseudomonas aeruginosa, have been spreading worldwide. -

Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy Using Machine Learning
Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy using Machine Learning Kate Wang et al. Supplemental Table S1. Drugs selected by Pharmacophore-based, ML-based and DL- based search in the FDA-approved drugs database Pharmacophore WEKA TF 1-Palmitoyl-2-oleoyl-sn-glycero-3- 5-O-phosphono-alpha-D- (phospho-rac-(1-glycerol)) ribofuranosyl diphosphate Acarbose Amikacin Acetylcarnitine Acetarsol Arbutamine Acetylcholine Adenosine Aldehydo-N-Acetyl-D- Benserazide Acyclovir Glucosamine Bisoprolol Adefovir dipivoxil Alendronic acid Brivudine Alfentanil Alginic acid Cefamandole Alitretinoin alpha-Arbutin Cefdinir Azithromycin Amikacin Cefixime Balsalazide Amiloride Cefonicid Bethanechol Arbutin Ceforanide Bicalutamide Ascorbic acid calcium salt Cefotetan Calcium glubionate Auranofin Ceftibuten Cangrelor Azacitidine Ceftolozane Capecitabine Benserazide Cerivastatin Carbamoylcholine Besifloxacin Chlortetracycline Carisoprodol beta-L-fructofuranose Cilastatin Chlorobutanol Bictegravir Citicoline Cidofovir Bismuth subgallate Cladribine Clodronic acid Bleomycin Clarithromycin Colistimethate Bortezomib Clindamycin Cyclandelate Bromotheophylline Clofarabine Dexpanthenol Calcium threonate Cromoglicic acid Edoxudine Capecitabine Demeclocycline Elbasvir Capreomycin Diaminopropanol tetraacetic acid Erdosteine Carbidopa Diazolidinylurea Ethchlorvynol Carbocisteine Dibekacin Ethinamate Carboplatin Dinoprostone Famotidine Cefotetan Dipyridamole Fidaxomicin Chlormerodrin Doripenem Flavin adenine dinucleotide -

Choline Esters As Absorption-Enhancing Agents for Drug Delivery Through Mucous Membranes of the Nasal, Buccal, Sublingual and Vaginal Cavities
J|A Europaisches Patentamt 0 214 ® ^KUw ^uroPean Patent O^ice (fl) Publication number: 898 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION @ Application number: 86401812.2 (3) Int. CI.4: A 61 K 47/00 @ Date of filing: 13.08.86 @ Priority: 16.08.85 US 766377 © Applicant: MERCK & CO. INC. 126, East Lincoln Avenue P.O. Box 2000 @ Date of publication of application : Rahway New Jersey 07065 (US) 18.03.87 Bulletin 87/12 @ Inventor: Alexander, Jose @ Designated Contracting States : 2909 Westdale Court CH DE FR GB IT LI NL Lawrence Kansas 66044 (US) Repta, A.J. Route 6, Box 100N Lawrence Kansas 66046 (US) Fix, Joseph A. 824 Mississippi Lawrence Kansas 66044 (US) @ Representative: Ahner, Francis et al CABINET REGIMBEAU 26, avenue Kleber F-75116 Paris (FR) |j) Choline esters as absorption-enhancing agents for drug delivery through mucous membranes of the nasal, buccal, sublingual and vaginal cavities. @ Choline esters are used as drug absorption enhancing agents for drugs which are poorly absorbed from the nasal, oral, and vaginal cavities. ! r ■ i ■ I njndesdruckerei Berlin 0 214 898 Description CHOLINE ESTERS AS ABSORPTION-ENHANCING AGENTS FOR DRUG DELIVERY THROUGH MUCOUS MEMBRANES OF THE NASAL, BUCCAL, SUBLINGUAL AND VAGINAL CAVITIES 5 BACKGROUND OF THE INVENTION The invention relates to a novel method and compositions for enhancing absorption of drugs from the nasal, buccal, sublingual and vaginal cavities by incorporating therein a choline ester absorption enhancing agent. The use of choline esters to promote nasal, buccal, sublingual and vaginal drug delivery offers several advantages over attempts to increase drug absorption from the gastrointestinal tract. -

Pharmaceutical Microbiology Table of Contents
TM Pharmaceutical Microbiology Table of Contents Pharmaceutical Microbiology ������������������������������������������������������������������������������������������������������������������������ 1 Strains specified by official microbial assays ������������������������������������������������������������������������������������������������ 2 United States Pharmacopeia (USP) �������������������������������������������������������������������������������������������������������������������������������������������2 European Pharmacopeia (EP) Edition 8�1 ���������������������������������������������������������������������������������������������������������������������������������5 Japanese Pharmacopeia (JP) ������������������������������������������������������������������������������������������������������������������������������������������������������7 Strains listed by genus and species �������������������������������������������������������������������������������������������������������������10 ATCC provides research and development tools and reagents as well as related biological material management services, consistent with its mission: to acquire, authenticate, preserve, develop, and distribute standard reference THE ESSENTIALS microorganisms, cell lines, and related materials for research in the life sciences� OF LIFE SCIENCE For over 85 years, ATCC has been a leading authenticate and further develop products provider of high-quality biological materials and services essential to the needs of basic and standards to the life -

Antimicrobial Resistance Benchmark 2020 Antimicrobial Resistance Benchmark 2020
First independent framework for assessing pharmaceutical company action Antimicrobial Resistance Benchmark 2020 Antimicrobial Resistance Benchmark 2020 ACKNOWLEDGEMENTS The Access to Medicine Foundation would like to thank the following people and organisations for their contributions to this report.1 FUNDERS The Antimicrobial Resistance Benchmark research programme is made possible with financial support from UK AID and the Dutch Ministry of Health, Welfare and Sport. Expert Review Committee Research Team Reviewers Hans Hogerzeil - Chair Gabrielle Breugelmans Christine Årdal Gregory Frank Fatema Rafiqi Karen Gallant Nina Grundmann Adrián Alonso Ruiz Hans Hogerzeil Magdalena Kettis Ruth Baron Hitesh Hurkchand Joakim Larsson Dulce Calçada Joakim Larsson Marc Mendelson Moska Hellamand Marc Mendelson Margareth Ndomondo-Sigonda Kevin Outterson Katarina Nedog Sarah Paulin (Observer) Editorial Team Andrew Singer Anna Massey Deirdre Cogan ACCESS TO MEDICINE FOUNDATION Rachel Jones The Access to Medicine Foundation is an independent Emma Ross non-profit organisation based in the Netherlands. It aims to advance access to medicine in low- and middle-income Additional contributors countries by stimulating and guiding the pharmaceutical Thomas Collin-Lefebvre industry to play a greater role in improving access to Alex Kong medicine. Nestor Papanikolaou Address Contact Naritaweg 227-A For more information about this publication, please contact 1043 CB, Amsterdam Jayasree K. Iyer, Executive Director The Netherlands [email protected] +31 (0) 20 215 35 35 www.amrbenchmark.org 1 This acknowledgement is not intended to imply that the individuals and institutions referred to above endorse About the cover: Young woman from the Antimicrobial Resistance Benchmark methodology, Brazil, where 40%-60% of infections are analyses or results. -
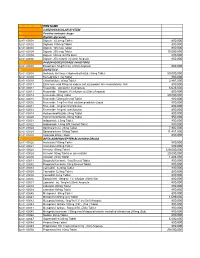
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

AMEG Categorisation of Antibiotics
12 December 2019 EMA/CVMP/CHMP/682198/2017 Committee for Medicinal Products for Veterinary use (CVMP) Committee for Medicinal Products for Human Use (CHMP) Categorisation of antibiotics in the European Union Answer to the request from the European Commission for updating the scientific advice on the impact on public health and animal health of the use of antibiotics in animals Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 29 October 2018 Adopted by the CVMP for release for consultation 24 January 2019 Adopted by the CHMP for release for consultation 31 January 2019 Start of public consultation 5 February 2019 End of consultation (deadline for comments) 30 April 2019 Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 19 November 2019 Adopted by the CVMP 5 December 2019 Adopted by the CHMP 12 December 2019 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Categorisation of antibiotics in the European Union Table of Contents 1. Summary assessment and recommendations .......................................... 3 2. Introduction ............................................................................................ 7 2.1. Background ........................................................................................................ -

Conjunctivitis Or Worse?
Red Eye in Dogs and CatS: Conjunctivitis or Worse? Tracy Revoir, DVM Senior Manager of Veterinary Support, Dechra Veterinary Products It should come as no surprise that conjunctivitis is Common Causes of Conjunctivitis the most common ophthalmic disorder in dogs and cats. But because the clinical signs of conjunctivitis If you do confirm conjunctivitis, the next step is can mimic those of more serious ophthalmic identifying the cause. If both eyes are affected and diseases (glaucoma and uveitis), it’s important to abnormal clinical signs are apparent in other body confirm your diagnosis. systems, think underlying systemic disease. If only one eye is affected, rule out infection, tear film What are important clues to the severity of the deficiencies, an irritant, anatomical abnormality, condition? With conjunctivitis, the inflammation or deeper ocular disease. should be limited to the conjunctiva. Hyperemic conjunctival vessels are superficial, branching, In dogs, conjunctivitis can result from anatomical and bright red. They are movable over the deeper disorders, irritants, infection (usually bacterial), or episcleral vessels and can be blanched with topical atopy. Most bacterial infections are secondary dilute phenylephrine. With glaucoma and uveitis, the conditions, most often to allergies. In cats, herpes- episcleral vessels are engorged; they are dark red, virus and Chlamydophila felis are the most common deep, straight, and immobile and do not blanch with causes of conjunctivitis. Atopy can also be topical dilute phenylephrine. With conjunctivitis, an issue in cats. the Schirmer tear test and intraocular pressures are normal. And the cornea should be clear and no aqueous flare should be present. The pupil and Addressing the Problem pupillary responses are normal and intraocular structures should be visible. -

Consideration of Antibacterial Medicines As Part Of
Consideration of antibacterial medicines as part of the revisions to 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) Section 6.2 Antibacterials including Access, Watch and Reserve Lists of antibiotics This summary has been prepared by the Health Technologies and Pharmaceuticals (HTP) programme at the WHO Regional Office for Europe. It is intended to communicate changes to the 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) to national counterparts involved in the evidence-based selection of medicines for inclusion in national essential medicines lists (NEMLs), lists of medicines for inclusion in reimbursement programs, and medicine formularies for use in primary, secondary and tertiary care. This document does not replace the full report of the WHO Expert Committee on Selection and Use of Essential Medicines (see The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization; 2019 (WHO Technical Report Series, No. 1021). Licence: CC BY-NC-SA 3.0 IGO: https://apps.who.int/iris/bitstream/handle/10665/330668/9789241210300-eng.pdf?ua=1) and Corrigenda (March 2020) – TRS1021 (https://www.who.int/medicines/publications/essentialmedicines/TRS1021_corrigenda_March2020. pdf?ua=1). Executive summary of the report: https://apps.who.int/iris/bitstream/handle/10665/325773/WHO- MVP-EMP-IAU-2019.05-eng.pdf?ua=1. -

Retention of Antibiotic Activity Against Resistant Bacteria
www.nature.com/scientificreports OPEN Retention of antibiotic activity against resistant bacteria harbouring amin ogl ycosid e‑ N‑ acetyl transferase enzyme by adjuvants: a combination of in‑silico and in‑vitro study Shamim Ahmed*, Sabrina Amita Sony, Md. Belal Chowdhury, Md. Mahib Ullah, Shatabdi Paul & Tanvir Hossain Interference with antibiotic activity and its inactivation by bacterial modifying enzymes is a prevailing mode of bacterial resistance to antibiotics. Aminoglycoside antibiotics become inactivated by aminoglycoside‑6′‑N‑acetyltransferase‑Ib [AAC(6′)‑Ib] of gram‑negative bacteria which transfers an acetyl group from acetyl‑CoA to the antibiotic. The aim of the study was to disrupt the enzymatic activity of AAC(6′)‑Ib by adjuvants and restore aminoglycoside activity as a result. The binding afnities of several vitamins and chemical compounds with AAC(6′)‑Ib of Escherichia coli, Klebsiella pneumoniae, and Shigella sonnei were determined by molecular docking method to screen potential adjuvants. Adjuvants having higher binding afnity with target enzymes were further analyzed in‑vitro to assess their impact on bacterial growth and bacterial modifying enzyme AAC(6′)‑Ib activity. Four compounds—zinc pyrithione (ZnPT), vitamin D, vitamin E and vitamin K‑exhibited higher binding afnity to AAC(6′)‑Ib than the enzyme’s natural substrate acetyl‑CoA. Combination of each of these adjuvants with three aminoglycoside antibiotics—amikacin, gentamicin and kanamycin—were found to signifcantly increase the antibacterial activity against the selected bacterial species as well as hampering the activity of AAC(6′)‑Ib. The selection process of adjuvants and the use of those in combination with aminoglycoside antibiotics promises to be a novel area in overcoming bacterial resistance. -

WO 2010/025328 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 4 March 2010 (04.03.2010) WO 2010/025328 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US2009/055306 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 28 August 2009 (28.08.2009) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/092,497 28 August 2008 (28.08.2008) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): FOR¬ GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, EST LABORATORIES HOLDINGS LIMITED [IE/ ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, —]; 18 Parliament Street, Milner House, Hamilton, TM), European (AT, BE, BG, CH, CY, CZ, DE, DK, EE, Bermuda HM12 (BM). -

Tackling Pristinamycin IIB Problems: Synthetic Studies Toward Some Fluorinated Analogs †
Proceeding Paper Tackling Pristinamycin IIB Problems: Synthetic Studies toward Some Fluorinated Analogs † Assia Chebieb * and Chewki Ziani-Cherif Department of Chemistry, University Abou-Bekr Belkaid of Tlemcen/Laboratory of Catalysis and Synthesis in Organic Chemistry LCSCO, 13000 Tlemcen, Algeria; [email protected] * Correspondence: [email protected] † Presented at the 24th International Electronic Conference on Synthetic Organic Chemistry, 15 November–15 December 2020; Available online: https://ecsoc-24.sciforum.net/. Abstract: Streptogramins are potent antibiotics against numerous highly resistant pathogens and therefore are used in last-resort human therapy. These antibiotics are formed of both A- and B-group compounds named pristinamycins that differ in their basic primary structures. Although pristina- mycin IIB is among the most interesting antibiotics in this family, it presents numerous problems related to its chemical structure, such as instability at most pH levels, weak solubility in water, and resistance by bacteria. As a response to the need for developing new antimicrobial agents, we have designed a new analog of pristinamycin IIB, based most importantly on the introduction of fluorine atoms. We conjectured indeed that the introduced modifications may solve the above-mentioned problems exhibited by pristinamycin IIB. Our multistep synthetic approach relies on few key reac- tions, namely a Wittig reaction, a Grubbs reaction, and dihydroxy, -difluoro API (Advanced Phar- maceutical Intermediate) synthesis Keywords: streptogramins; pristinamycins IIB; fluorine Citation: Chebieb, A.; Ziani-Cherif, C. Tackling Pristinamycin IIB Problems: Synthetic Studies toward Some Fluorinated Analogs. Chem. The first antibiotic mixture of streptogramin antibiotics was isolated from the pro- Pro. 2021, 3, 107. https://doi.org/ ducer strain Streptomyces graminofaciens from a soil sample in Texas [1].