Adrenal Gland, Cortex – Hypertrophy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Adrenal Capsule Is a Signaling Center Controlling Cell Renewal and Zonation Through Rspo3
Downloaded from genesdev.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION The permanent cortex is formed through recruitment of The adrenal capsule is a capsular cells in a process that involves SHH signaling signaling center controlling (King et al. 2009). By E17.5, steroidogenic cells have adopted specific expression profiles, with the outermost cell renewal and zonation cell layers (zona glomerulosa [ZG]) producing enzymes Rspo3 that are required for mineralocorticoid production (e.g., through CYP11B2), and deeper layers (zona fasciculata [ZF]) Valerie Vidal,1,2,3,9 Sonia Sacco,1,2,3,9 expressing genes involved in glucocorticoid synthesis Ana Sofia Rocha,1,2,3,8 Fabio da Silva,1,2,3 (Cyp11b1). In humans, but not rodents, a third layer (zona reticularis) can be distinguished that produces an- Clara Panzolini,1,2,3 Typhanie Dumontet,4,5 1,2,3 6 drogens and is located close to the medulla. Several lines Thi Mai Phuong Doan, Jingdong Shan, of evidence suggest that β-catenin plays an important Aleksandra Rak-Raszewska,6 Tom Bird,7 role in adrenal zonation and maintenance. Activation of Seppo Vainio,6 Antoine Martinez,4,5 the β-catenin pathway is restricted to the ZG (Kim et al. and Andreas Schedl1,2,3 2008; Walczak et al. 2014), and ectopic expression leads to the activation of ZG markers in ZF cells (Berthon 1Institute of Biology Valrose, Université de Nice-Sophia, F-06108 et al. 2010). Moreover, β-catenin seems to bind to and con- Nice, France; 2UMR1091, Institut National de la Santé et de la trol the expression of At1r, a gene specifically expressed Recherche Médicale, F-06108 Nice, France; 3CNRS, UMR7277, within the ZG (Berthon et al. -

Histogenesis of Suprarenal Glands at Different Gestational Age Groups
ORIGINAL ARTICLE ASIAN JOURNAL OF MEDICAL SCIENCES Histogenesis of suprarenal glands at different gestational age groups Ravindra Kumar Boddeti1, Subhadra Devi Velichety2 1Lecturer, 2Professor and Head, Department of Anatomy, Sri Padmavathi Medical College for Women, Sri Venkateswara Institute of Medical Sciences, SVIMS University, Tirupathi, Andhra Pradesh, India Submitted: 22-02-2019 Revised: 10-03-2019 Published: 01-05-2019 ABSTRACT Background: The human foetal suprarenal gland is structurally variant from its adult Access this article online counterpart. The most distinctive features of human foetal suprarenal gland and histologically Website: unique foetal zone, was described first by Elliott and Armour in 1911. After the first trimester, the centrally located foetal zone accounts for most of the foetal adrenal mass. The outer zone http://nepjol.info/index.php/AJMS of the foetal suprarenal gland is called the “definitive zone or neo cortex”; this zone likely DOI: 10.3126/ajms.v10i3.22820 gives rise to the adult adrenal glomerulosa. A third zone called “transitional zone”, lies just E-ISSN: 2091-0576 2467-9100 between the neocortex and foetal zone and is believed to develop into the zona fasciculata. P-ISSN: Aims and Objectives: The current study was designed to study the histogenesis of suprarenal glands at different gestational age groups. Materials and Methods: Twenty-eight formalin preserved dead embryos and foetuses of both sexes, were obtained from the Govt. Maternity Hospital & S.V.Medical College, Tirupati, Andhra Pradesh, India. Specimens were grouped according to their gestational age groups (A,B,C,D) A= 0-12 weeks, B= 13-24 weeks, C= 25-36 weeks and D= more than 36 weeks of gestation. -

Adrenal Gland Hormones
CHAPTER 8 Adrenal Gland Hormones Devra K. Dang, PharmD, BCPS, CDE, FNAP | Trinh Pham, PharmD, BCOP | Jennifer J. Lee, PharmD, BCPS, CDE LEARNING OBJECTIVES KEY TERMS AND DEFINITIONS After completing this chapter, you should be able to ACTH (adrenocorticotropic hormone) — a hormone produced 1. Identify the hormones produced by the adrenal glands by the pituitary gland that stimulates 2. Describe the functions of mineralocorticoids and glucocorticoids in the body the adrenal cortex to produce glucocorticoids, mineralocorticoids, 3. Recognize the signs and symptoms of adrenal insuffi ciency and androgens. PART 4. Describe the pharmacological treatment of patients with acute and chronic adrenal Addison ’ s disease — a disorder insuffi ciency in which the adrenal glands do not produce enough steroid hormones. 3 5. Recognize the signs and symptoms of Cushing ’ s syndrome and the result of too Adenoma — a benign much cortisol (noncancerous) tumor of glandular 6. Describe the pharmacologic and nonpharmacologic management of patients with origin. Cushing ’ s syndrome Adrenal insuffi ciency — a term 7. List management strategies for administration of glucocorticoid and mineralocorti- referring to a defi ciency in the levels of adrenal hormones. coid therapy to avoid development of adrenal disorders Aldosterone — the hormone produced by the adrenal glands that regulates the balance of sodium, he adrenal glands are an integral part of the endocrine system, secreting water, and potassium concentrations in the body. T hormones that act throughout the body to regulate functions and promote Corticotropin-releasing homeostasis. In addition to the neurotransmitters epinephrine and norepineph- hormone (CRH) — a hormone rine, the corticosteroids secreted by the adrenal glands are vital to a wide released by the hypothalamus that variety of physiological processes. -

Hypothalamushypothalamus -- Pituitarypituitary -- Adrenaladrenal Glandsglands
HypothalamusHypothalamus -- pituitarypituitary -- adrenaladrenal glandsglands Magdalena Gibas-Dorna MD, PhD Dept. of Physiology University of Medical Sciences Poznań, Poland Hypothalamus - general director of the hormone system. At every moment, the hypothalamus analyses messages coming from: the brain and different regions of the body. Homeostatic functions of hypothalamus include maintaining a stable body temperature, controlling food intake, controlling blood pressure, ensuring a fluid balance, and even proper sleep patterns. Cell bodies of neurons that produce releasing/inhibiting hormones Hypothalamus HypothalamusHypothalamus releases Arterial flow Primary capillaries in median eminence hormones at Long Releasing Portal hormones Anterior veins median eminence pituitary hormone Releasing/ inhibiting hormones and sends to anterior pituitary ANTERIOR PITUITARY via portalportal veinvein. Secretory cells that produce anterior pituitary hormones Anterior pituitary hormones Venous outflow Gonadotropic Thyroid- Proactin hormones stimulating ACTH Growth (FSH and LH) hormone hormone ControlControl ofof pituitarypituitary hormonehormone secretionsecretion byby hypothalamushypothalamus • Secretion by the anterioranterior pituitarypituitary is controlled by hormones called hypothalamic releasing hormones and inhibitory hormones conducted to the anterior pituitary through hypothalamichypothalamic -- hypophysialhypophysial portalportal vesselsvessels .. • PosteriorPosterior pituitarypituitary secrets two hormones, which are synthesized within cell -
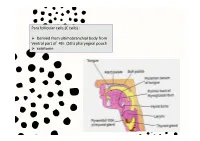
Para Follicular Cells (C Cells)
Para follicular cells (C cells) : Derived from ultimobranchial body from Ventral part of 4th (5th) pharyngeal pouch calcitonin Thyroglossal cyct: path of thyroid descending Position of occur: Inf. To the body of hyoid 50% Base of tongue Close to thyroid cartilage Thyroglossal fistula Abberant thyroid tissue: path of thyroid descending Base of tongue Histology of the thyroid gland Thyroid gland: parafollicular cells: Capsule / trabeculae Follicles / reticular fiber / Decreased Ca+ in blood by 2 ways: basal lamina Follicular cells/ basal lamina 1. Transport Ca from blood to musculoskeletal system parafollicular cells 2. Prevent bone absorption by osteoclast cells Colloid (hormone storage) Produce energy & temperature for body activity Histology of the thyroid gland Thyroid hormones: Tri-iodothyronin Tetra-iodothyronin Calcitonin Fenestrated capillary Synthesis and secretion of thyroid hormones T3 and T4 Synthesis and secretion of thyroid hormones T3 and T4 Synthesis and mechanism of action of calcitonin Gravies disease (exophthalmic goiter / toxic goiter): excessive amounts of thyroid hormones are released into the circulation detectable levels of autoantibodies abnormal immunoglobulins (IgG) bind to the TSH receptor in-creased thyroid hormone secretion Because of negative feedback, the levels of TSH in the circulation are usually normal Hypertrophy thyroid hormone Is abnormally high range increased metabolism Features: weight loss / excessive sweating / tachycardia /nervousness / protrusion of the eyeballs / retraction of the -

A Histocytological Study of Age Changes in the Canine Adrenal Gland Studied by Light and Electron Microscopy Ronald Loral Hullinger Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1968 A histocytological study of age changes in the canine adrenal gland studied by light and electron microscopy Ronald Loral Hullinger Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Animal Structures Commons, and the Veterinary Anatomy Commons Recommended Citation Hullinger, Ronald Loral, "A histocytological study of age changes in the canine adrenal gland studied by light and electron microscopy" (1968). Retrospective Theses and Dissertations. 3674. https://lib.dr.iastate.edu/rtd/3674 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dlsseriaUon has been microfihned exactly as received 68-14,799 HULLINGER, D.V.M., Ronald Loral, 1941- A HISTOCYTOLOGICAL STUDY OF AGE CHANGES IN THE CANINE ADRENAL GLAND STUDIED BY LIGHT AND ELECTRON MICROSCOPY. Iowa State University, Ph,D., 1968 Anatomy University Microfilms, Inc., Ann Arbor, Michigan A HISTOCYTOLOGICAL STUDY OF AGE CHANGES IN THE CANINE ADRENAL GLAND STUDIED BY LIGHT AND ELECTRON MICROSCOPY by ' Ronald Loral Hullinger , D. v,'- A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Veterinary Anatomy Approved; Signature was redacted for privacy. In Charge of Majpr^Work Signature was redacted for privacy. .rtment Signature was redacted for privacy. -
The Endocrine System
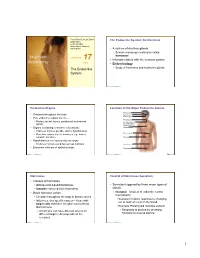
PowerPoint® Lecture Slides The Endocrine System: An Overview prepared by Leslie Hendon University of Alabama, Birmingham • A system of ductless glands • Secrete messenger molecules called hormones C H A P T E R 17 • Interacts closely with the nervous system Part 1 • Endocrinology The Endocrine • Study of hormones and endocrine glands System Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Endocrine Organs Location of the Major Endocrine Glands Pineal gland • Scattered throughout the body Hypothalamus Pituitary gland • Pure endocrine organs are the … Thyroid gland • Pituitary, pineal, thyroid, parathyroid, and adrenal Parathyroid glands glands (on dorsal aspect of thyroid gland) • Organs containing endocrine cells include: Thymus • Pancreas, thymus, gonads, and the hypothalamus Adrenal glands • Plus other organs secrete hormones (eg., kidney, stomach, intestine) Pancreas • Hypothalamus is a neuroendocrine organ • Produces hormones and has nervous functions Ovary (female) Endocrine cells are of epithelial origin • Testis (male) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 17.1 Hormones Control of Hormones Secretion • Classes of hormones • Amino acid–based hormones • Secretion triggered by three major types of • Steroids—derived from cholesterol stimuli: • Basic hormone action • Humoral—simplest of endocrine control mechanisms • Circulate throughout the body in blood vessels • Secretion in direct response to changing • Influences only specific tissues— those with ion or nutrient levels in the blood target cells that have receptor molecules for that hormone • Example: Parathyroid monitors calcium • A hormone can have different effects on • Responds to decline by secreting different target cells (depends on the hormone to reverse decline receptor) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. -

Adrenal & Gonadal Hormones Layers of Adrenal Cortex
Adrenal & Gonadal Hormones Topics for today: •Adrenal cortex hormone •Adrenal medulla hormones •Hormone control of organs •Steroid hormone synthesis •Vitamin D3 • Estrogens and Progesterone Layers of adrenal cortex zona glomerulosa zona fasiculata zona reticularis 1 Hormones of adrenal cortex zona glomerulosa zona fasiculata O zona reticularis HO dehydroepiandrosterone Androgens – dehydroepiandrosterone • increased protein synthesis DHEA is weak androgen with • masculinizing effects in almost no effect in male but has female (hypersecretion) masculinizing effects in females Adrenal medulla (interior) • Composed of modified post-synaptic sympathetic neurons • Releases mostly epinephrine. • Has effects similar to those triggered by sympathetic nervous system Adrenal medulla hormones HO HO -CH-CH2 - N-CH3 epinephrine OH H Effects of epinephrine: • causes elevated blood glucose level • stimulates glycolysis & fatty acid use • increases cardiac output & blood pres • shifts blood flow to skeletal muscle • increases rate and depth of respiration 2 Organ responses to Epinephrine Causes glycogen Causes fatty acid release degradation in muscle from adipose tissue fatty glycogen acids trigly- cerides lactate glycerol muscle lactate adipose tissue Causes release of glycogeno lysis glucose from liver glucose ...elevated plasma level liver Hormone action time Epinephrine is in group of fast-acting hormones Fast-acting Hormones Slow-acting Hormones • Norepinephrine • Throxine • Epinephrine • Cortisol • Insulin • Growth hormone • glucagon • Estrogens -

The Mononuclear Phagocyte System of the Mouse Defined By
Proc. Nal. Acad. Sci. USA Vol. 81, pp. 4174-4177, July 1984 Immunology The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: Macrophages of endocrine organs (pituitary/adrenal cortex/corpus luteum/testis) DAVID A. HUME*, DAVID HALPINtt, HARRY CHARLTONt, AND SIAMON GORDON§ *Department of Medicine and Cliiical Science, John Curtin School of Medical Research, Canberra, Australia; and §Sir William Dunn School of Pathology and tDepartment of Anatomy, Oxford University, South Parks Road, Oxford OX1 3RE, United Kingdom Communicated by Zanvil A. Cohn, March 19, 1984 ABSTRACT Macrophages of endocrine organs have been tains an abundant F4/80' population (Fig. 1A). The bodies identified by immunohistochemical localization of the macro- of the labeled cells are wrapped around capillaries or line phage-specific antigen F4/80. P4180' cells line vascular sinus- vascular sinuses, but membrane processes extend into the es-and capillaries in anterior and posterior pituitary, adrenal surrounding tissue so that many cells have adjacent F4/80+ cortex, corpus luteum, parathyroid, pineal gland, and islets of material. By comparison with the zona glomerulosa, the ra- Langerhans. In testis approximately 20% of interstitial cells diating sinuses of the zona fasciculata are less heavily popu- are F4/80'. F4/80' cells infiltrate corpus luteum in increased lated with F4/80+ cells. At low power they appear absent, numbers during luteolysis. but closer examination shows that the cells are much more extensively spread than in zona glomerulosa with decreased The mononuclear phagocyte system is a group of cells that stain intensity (Fig. 1A). Adrenal cortical sinusoidal F4/80+ consists of bone marrow precursors, blood monocytes, and cells are smaller and more flattened (in the sinus wall) than tissue macrophages (1). -

SSAT ABSITE Review: Endocrine Adrenal, Thyroid, Parathyroid
SSAT ABSITE Review: Endocrine Adrenal, Thyroid, Parathyroid Douglas Cassidy, MD MGH Surgical Education Research and Simulation Fellow @DJCSurgEd https://www.youtube.com/c/surgedvidz 1/22/2020 1 Content Outline • Adrenal: • Parathyroid • Anatomy and Physiology • Anatomy • Incidentalomas • Calcium Homeostasis • Adrenal Cortical Carcinoma • Primary Hyperparathyroidism • Multiple Endocrine Neoplasia • Head and Neck: • Thyroid • Anatomy • Physiology • Neck Dissections • Thyroid Nodules + Ultrasound • Head and Neck Cancers • Thyroid Cancers • Hypo- and Hyper-thyroidism Adrenal Anatomy • Paired RP endocrine glands above superior pole of kidneys • Arterial: • Superior suprarenal from inferior phrenic • Middle suprarenal from abdominal aorta • Inferior suprarenal from renal artery • Venous: • Left adrenal vein drains into the left renal vein • Right adrenal vein drains directly into the IVC Adrenal Incidentalomas: • Evaluation: • Is the mass functioning or non-functioning? • Is the mass benign or malignant? • If malignant, is it primary or secondary? Adrenal Incidentalomas: • Functional Masses: • Adrenal Cortex: • Zona Glomerulosa -- Aldosterone • Zona Fasciculata -- Cortisol • Zona Reticularis -- Androgens • Adrenal Medulla: Catecholamines • Epinephrine / Norepinephrine Aldosteronomas • Function: ↑Na+ absorption and K+ secretion in the distal tubule • ↑H+ excretion in the collecting duct • Labs: ↑Na+, ↓K+, METABOLIC ALKALOSIS • Presentation: uncontrolled / drug- resistant HTN, sxs of low K+ (cramps, weakness) • DDx: • 1°: Adenoma, Hyperplasia, -

Cerebral Cortex Hypothalamus Anterior Pituitary Adrenal Gland
Endocrine System Cerebral cortex Hypothalamus Releasing Hormones Anterior Pituitary Glucose Low Ca2+ ACTH TSH Adrenal Low Blood Pressure Thyroid Pancreas Parathyroid gland Aldosterone Cortisol T3/T4 Insulin PTH Target tissue Pituitary Gland Hypothalamus Pituitary Stalk Hypothalamic Axons Anterior Pituitary Posterior Pituitary Axon termini release Cluster of hormome- hormones secreting cells Anterior Pituitary Posterior Pituitary • Growth Hormone (GH) • Oxytocin • Adrenocorticotrophic Hormone • Vasopressin (ACTH) • Thyroid-Stimulating Hormone (TSH) • Prolactin • Leutenizing Hormone (LH) • Follicle-Stimulating Hormone (FSH) Pituitary Optic Chiasm Hypothalamus Pituitary Stalk Posterior Pituitary Anterior Pituitary Anterior Pituitary Acidophil Basophil Chromophobe Capillary Reticulin Fibers Hypothalamus hormones that regulate release of pituitary hormones • Growth Hormone Releasing Hormone (GHRH) -> GH • Corticotropin Releasing Hormone (CRH) -> ACTH • Thryotropin Releasing Hormone (TRH) -> TSH • Gonadotropin Releasing Hormone -> FSH and LH Hypothalamus 1. Neurons synthesize hormone-releasing hormones 2. Neurons release hormone- releasing hormones into primary capillary plexus. Portal Vein 3. Hormone-releasing hormones diffuse out of secondary plexus to stimulate acidophils and basophils. Anterior Pituitary 4. Hormones from acidophils and basophils diffuse into secondary plexus and distribute throughout the body. Anterior Pituitary Fenestrated Endothelium Somatotroph Secretory Granule Hypothalamus Pituitary Stalk Hypothalamic Axons Anterior Pituitary -
TITLE: Aldosterone and Renin PRESENTER: Jieli Shirley Li MD, Phd
TITLE: Aldosterone and Renin PRESENTER: Jieli Shirley Li MD, PhD Slide 1: Hello, my name is Jieli Shirley Li. I am an assistant professor and lab director at Ohio State University Wexner Medical Center. Welcome to this Pearl of Laboratory Medicine on “Aldosterone and Renin” Slide 2: Anatomically, the adrenal glands are divided into two distinct parts, the medulla, the inner layer and the cortex, the outer layer. The cortex is further divided into three zones. The outermost zona glomerulosa, which produces mineralocorticoids; the zona fasciculata, which is responsible for glucocorticoid production; and the inner zona reticularis, which synthesizes androgens. The cortex makes up about 80% to 90% of the adrenal gland. The medulla stores and secretes catecholamines. Slide 3: The hormones of the adrenal cortex are steroid derivatives, synthesized from cholesterol. Cholesterol travels across the mitochondrial membrane. In the zona glomerulosa, 3β- hydroxysteroid dehydrogenase, 21-hydroxylase and 11β-hydroxylase are the major enzymes which are involved in the aldosterone synthesis. In addition, aldosterone is synthesized by aldosterone synthase, an enzyme encoded by CYP11B2. Aldosterone synthase expresses almost entirely in the adrenal cortex and exclusively in the zona glomerulosa layer. Isolated © 2016 Clinical Chemistry Pearls of Laboratory Medicine Aldosterone and Renin deficiencies of aldosterone biosynthesis could be caused by inactivating mutations in the CYP11B2 gene. Slide 4: The chief mineralocorticoid is aldosterone. It plays an important role in maintaining blood volume, pressure, pH, and electrolyte balance. It promotes the reabsorption of sodium, and meanwhile increases potassium and hydrogen ion excretion which increases blood pH. Therefore, in hyperaldosteronism, the overproduction of aldosterone leads to the retention of sodium and loss of potassium in the body, resulting in high hypertension, hypokalemia and alkalosis.