The Endocrine System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expression Pattern of Delta-Like 1 Homolog in Developing Sympathetic Neurons and Chromaffin Cells
Published in "Gene Expression Patterns 30: 49–54, 2018" which should be cited to refer to this work. Expression pattern of delta-like 1 homolog in developing sympathetic neurons and chromaffin cells ∗ Tehani El Faitwria,b, Katrin Hubera,c, a Institute of Anatomy & Cell Biology, Albert-Ludwigs-University Freiburg, Albert-Str. 17, 79104, Freiburg, Germany b Department of Histology and Anatomy, Faculty of Medicine, Benghazi University, Benghazi, Libya c Department of Medicine, University of Fribourg, Route Albert-Gockel 1, 1700, Fribourg, Switzerland ABSTRACT Keywords: Delta-like 1 homolog (DLK1) is a member of the epidermal growth factor (EGF)-like family and an atypical notch Sympathetic neurons ligand that is widely expressed during early mammalian development with putative functions in the regulation Chromaffin cells of cell differentiation and proliferation. During later stages of development, DLK1 is downregulated and becomes DLK1 increasingly restricted to specific cell types, including several types of endocrine cells. DLK1 has been linked to Adrenal gland various tumors and associated with tumor stem cell features. Sympathoadrenal precursors are neural crest de- Organ of Zuckerkandl rived cells that give rise to either sympathetic neurons of the autonomic nervous system or the endocrine Development ffi Neural crest chroma n cells located in the adrenal medulla or extraadrenal positions. As these cells are the putative cellular Phox2B origin of neuroblastoma, one of the most common malignant tumors in early childhood, their molecular char- acterization is of high clinical importance. In this study we have examined the precise spatiotemporal expression of DLK1 in developing sympathoadrenal cells. We show that DLK1 mRNA is highly expressed in early sympa- thetic neuron progenitors and that its expression depends on the presence of Phox2B. -

The Morphology, Androgenic Function, Hyperplasia, and Tumors of the Human Ovarian Hilus Cells * William H
THE MORPHOLOGY, ANDROGENIC FUNCTION, HYPERPLASIA, AND TUMORS OF THE HUMAN OVARIAN HILUS CELLS * WILLIAM H. STERNBERG, M.D. (From the Department of Pathology, School of Medicine, Tulane University of Louisiana and the Charity Hospital of Louisiana, New Orleans, La.) The hilus of the human ovary contains nests of cells morphologically identical with testicular Leydig cells, and which, in all probability, pro- duce androgens. Multiple sections through the ovarian hilus and meso- varium will reveal these small nests microscopically in at least 8o per cent of adult ovaries; probably in all adult ovaries if sufficient sections are made. Although they had been noted previously by a number of authors (Aichel,l Bucura,2 and von Winiwarter 3"4) who failed to recog- nize their significance, Berger,5-9 in 1922 and in subsequent years, pre- sented the first sound morphologic studies of the ovarian hilus cells. Nevertheless, there is comparatively little reference to these cells in the American medical literature, and they are not mentioned in stand- ard textbooks of histology, gynecologic pathology, nor in monographs on ovarian tumors (with the exception of Selye's recent "Atlas of Ovarian Tumors"10). The hilus cells are found in clusters along the length of the ovarian hilus and in the adjacent mesovarium. They are, almost without excep- tion, found in contiguity with the nonmyelinated nerves of the hilus, often in intimate relationship to the abundant vascular and lymphatic spaces in this area. Cytologically, a point for point correspondence with the testicular Leydig cells can be established in terms of nuclear and cyto- plasmic detail, lipids, lipochrome pigment, and crystalloids of Reinke. -

Thyroid Gland Parathyroid Glands
Human Physiology Course Thyroid Gland Parathyroid Glands Assoc. Prof. Mária Pallayová, MD, PhD [email protected] Department of Human Physiology, UPJŠ LF April 13, 2020 (10th week – Summer Semester 2019/2020) Hormones and Functions Thyroid gland Parathyroids Thymus Adrenal glands Endocrine pancreas Ovaries, Testes Pineal Pituitary Hypothalamus-Pituitary Axis GIT, adipose tissue, brain, heart, kidney, ... Hormones and Functions Thyroid gland Parathyroids Thymus Adrenal glands Endocrine pancreas Ovaries, Testes Pineal Pituitary Hypothalamus-Pituitary Axis GIT, adipose tissue, brain, heart, kidney, ... Lecture Outline Functional anatomy of the thyroid gland Synthesis, secretion, and metabolism of the thyroid hormones The mechanism of thyroid action Role of the thyroid hormones in development, growth, and metabolism Thyroid hormone deficiency and excess in adults Physiology of the parathyroids Functional Anatomy of the Thyroid Gland two lobes + isthmus (just below the cricoid cartilage) attached to the trachea by connective tissue A normal THGL in a healthy adult weighs about 15-20 g. Functional Anatomy of the Thyroid Gland arterial blood supply: from a superior and an inferior thyroid a., which arise from the external carotid and subclavian a., respectively. venous blood supply: a series of thyroid veins drain into the ext. jugular and innominate vv. ( a rich blood supply to the thyroid gland w/ a higher rate of blood flow per gram than even that of the kidneys). innervation: adrenergic innervation from the cervical ganglia; cholinergic innervation from the n. vagus (regulation of vasomotor function to increase the delivery of TSH, iodide, and metabolic substrates to the THGL). Functional Anatomy of the Thyroid Gland The colloid (a thick, gel-like substance) is a solution composed primarily of thyroglobulin (10-25% the high viscosity), a large protein that is a storage form of the thyroid hormones. -
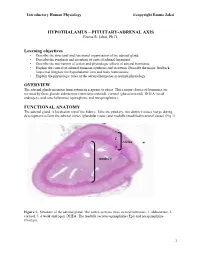
HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Learning Objectives OVERVIEW FUNCTIONAL ANATOMY
Introductory Human Physiology ©copyright Emma Jakoi HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Emma R. Jakoi, Ph.D. Learning objectives • Describe the structural and functional organization of the adrenal gland. • Describe the synthesis and secretion of cortical adrenal hormones. • Describe the mechanism of action and physiologic effects of adrenal hormones. • Explain the control of adrenal hormone synthesis and secretion. Describe the major feedback loops that integrate the hypothalamic axis and body homeostasis. • Explain the physiologic roles of the adrenal hormones in normal physiology. OVERVIEW The adrenal glands maintain homeostasis in response to stress. Three major classes of hormones are secreted by these glands: aldosterone (mineralocorticoid), cortisol (glucocorticoid), DHEA (weak androgen), and catecholamines (epinephrine and norepinephrine). FUNCTIONAL ANATOMY The adrenal gland is located on top of the kidney. Like the pituitary, two distinct tissues merge during development to form the adrenal cortex (glandular tissue) and medulla (modified neuronal tissue) (Fig 1). 1 2 cortex 3 medulla Figure 1. Structure of the adrenal gland. The cortex secretes three steroid hormones: 1. aldosterone, 2. cortisol, 3. a weak androgen, DHEA. The medulla secretes epinephrine (Epi) and norepinephrine (NorEpi). 1 Introductory Human Physiology ©copyright Emma Jakoi MINERALOCORTICOIDS The major mineralocorticoid in humans is aldosterone. Aldosterone is NOT under the hypothalamus- pituitary control and does not mediate a negative feedback to this axis. Aldosterone secretion is increased by the vasoconstrictor, angiotensin II, and by elevated plasma K+ concentration. Elevated plasma Na+ inhibits the secretion of aldosterone. Aldosterone, acts in the kidney to promote secretion of K+ into the urine from the blood and the reabsorption of Na+ from the urine into the blood. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Some Fundamentals of Gonadal Development and Function Richmond W
Henry Ford Hospital Medical Journal Volume 8 | Number 3 Article 8 9-1960 Some Fundamentals Of Gonadal Development And Function Richmond W. Smith Jr. Raymond C. Mellinger Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Smith, Richmond W. Jr. and Mellinger, Raymond C. (1960) "Some Fundamentals Of Gonadal Development And Function," Henry Ford Hospital Medical Bulletin : Vol. 8 : No. 3 , 324-344. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol8/iss3/8 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. For more information, please contact [email protected]. SOME FUNDAMENTALS OF GONADAL DEVELOPMENT AND FUNCTION* RICHMOND W. SMITH, JR., M.D.** AND RAYMOND C. MELLINGER, M.D.** The traditional division of animal life into male and female forms is based on obvious biological differences, but these distinctions become less striking when we realize that life, in a sheer physico-chemical sense, is a spectrum of sexuality that maleness and femaleness are relative terms. Our conceptual devotion to a two compartment universe is apparent in many areas of life, sociologic, moral, legal, spiritual or biologic. Although reproductive obligations remain clear, albeit increasingly restricted, man's greater social sophistication is molding an order in which underlying biological distinctions of the two sexes are sometimes obscured by the potent solvents of culture, leisure and intellect. -

The Adrenal Capsule Is a Signaling Center Controlling Cell Renewal and Zonation Through Rspo3
Downloaded from genesdev.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION The permanent cortex is formed through recruitment of The adrenal capsule is a capsular cells in a process that involves SHH signaling signaling center controlling (King et al. 2009). By E17.5, steroidogenic cells have adopted specific expression profiles, with the outermost cell renewal and zonation cell layers (zona glomerulosa [ZG]) producing enzymes Rspo3 that are required for mineralocorticoid production (e.g., through CYP11B2), and deeper layers (zona fasciculata [ZF]) Valerie Vidal,1,2,3,9 Sonia Sacco,1,2,3,9 expressing genes involved in glucocorticoid synthesis Ana Sofia Rocha,1,2,3,8 Fabio da Silva,1,2,3 (Cyp11b1). In humans, but not rodents, a third layer (zona reticularis) can be distinguished that produces an- Clara Panzolini,1,2,3 Typhanie Dumontet,4,5 1,2,3 6 drogens and is located close to the medulla. Several lines Thi Mai Phuong Doan, Jingdong Shan, of evidence suggest that β-catenin plays an important Aleksandra Rak-Raszewska,6 Tom Bird,7 role in adrenal zonation and maintenance. Activation of Seppo Vainio,6 Antoine Martinez,4,5 the β-catenin pathway is restricted to the ZG (Kim et al. and Andreas Schedl1,2,3 2008; Walczak et al. 2014), and ectopic expression leads to the activation of ZG markers in ZF cells (Berthon 1Institute of Biology Valrose, Université de Nice-Sophia, F-06108 et al. 2010). Moreover, β-catenin seems to bind to and con- Nice, France; 2UMR1091, Institut National de la Santé et de la trol the expression of At1r, a gene specifically expressed Recherche Médicale, F-06108 Nice, France; 3CNRS, UMR7277, within the ZG (Berthon et al. -

Histogenesis of Suprarenal Glands at Different Gestational Age Groups
ORIGINAL ARTICLE ASIAN JOURNAL OF MEDICAL SCIENCES Histogenesis of suprarenal glands at different gestational age groups Ravindra Kumar Boddeti1, Subhadra Devi Velichety2 1Lecturer, 2Professor and Head, Department of Anatomy, Sri Padmavathi Medical College for Women, Sri Venkateswara Institute of Medical Sciences, SVIMS University, Tirupathi, Andhra Pradesh, India Submitted: 22-02-2019 Revised: 10-03-2019 Published: 01-05-2019 ABSTRACT Background: The human foetal suprarenal gland is structurally variant from its adult Access this article online counterpart. The most distinctive features of human foetal suprarenal gland and histologically Website: unique foetal zone, was described first by Elliott and Armour in 1911. After the first trimester, the centrally located foetal zone accounts for most of the foetal adrenal mass. The outer zone http://nepjol.info/index.php/AJMS of the foetal suprarenal gland is called the “definitive zone or neo cortex”; this zone likely DOI: 10.3126/ajms.v10i3.22820 gives rise to the adult adrenal glomerulosa. A third zone called “transitional zone”, lies just E-ISSN: 2091-0576 2467-9100 between the neocortex and foetal zone and is believed to develop into the zona fasciculata. P-ISSN: Aims and Objectives: The current study was designed to study the histogenesis of suprarenal glands at different gestational age groups. Materials and Methods: Twenty-eight formalin preserved dead embryos and foetuses of both sexes, were obtained from the Govt. Maternity Hospital & S.V.Medical College, Tirupati, Andhra Pradesh, India. Specimens were grouped according to their gestational age groups (A,B,C,D) A= 0-12 weeks, B= 13-24 weeks, C= 25-36 weeks and D= more than 36 weeks of gestation. -

Downloaded from Bioscientifica.Com at 09/30/2021 12:01:31AM Via Free Access 706 D BARON and Others · Foxl2 and Rainbow Trout Gonad Differentiation
705 An evolutionary and functional analysis of FoxL2 in rainbow trout gonad differentiation Daniel Baron1, Julie Cocquet2, Xuhua Xia3, Marc Fellous2, Yann Guiguen1 and Reiner A Veitia2 1INRA-SCRIBE, Campus de Beaulieu, 35042 Rennes Cedex, France 2INSERM E0021 & V361, Génomique fonctionnelle du Développement, Hôpital Cochin, 123 Bd de Port Royal, Paris, France 3Department of Biology and the Center for Advanced Research in Environmental Genomics, University of Ottawa, Ottawa, Ontario, Canada (Requests for offprints should be addressed to R A Veitia; Email: [email protected]) Abstract FOXL2 is a forkhead transcription factor involved in ovarian development and function. Here, we have studied the evolution and pattern of expression of the FOXL2 gene and its paralogs in fish. We found well conserved FoxL2 sequences (FoxL2a) and divergent genes, whose forkhead domains belonged to the class L2 and were shown to be paralogs of the FoxL2a sequences (named FoxL2b). In the rainbow trout, FoxL2a and FoxL2b were specifically expressed in the ovary, but displayed different temporal patterns of expression. FoxL2a expression correlated with the level of aromatase, the key enzyme in estrogen production, and an estrogen treatment used to feminize genetically male individuals elicited the up-regulation of both paralogs. Conversely, androgens or an aromatase inhibitor down-regulated FoxL2a and FoxL2b in females. We speculate that there is a direct link between estrogens and FoxL2 expression in fish, at least during the period where the identity of the gonad is sensitive to hormonal treatments. Journal of Molecular Endocrinology (2004) 33, 705–715 Introduction been detected (Cocquet et al. 2002, Pannetier et al. -

The Fine Structure of the Parathyroid Gland*
The Fine Structure of the Parathyroid Gland* BY JERRY STEVEN TRIER, M.D. (From the Department of Anatomy, University of Washington School of Medicine, Seattle) PLATES 3 TO 10 (Received for publication, July 29, 1957) ABSTRACT The fine structure of the parathyroid of the macaque is described, and is cor- related with classical parathyroid cytology as seen in the light microscope. The two parenchymal cell types, the chief cells and the oxyphil cells, have been recognized in electron mierographs. The chief cells contain within their cyto- plasm mitochondria, endoplasmic reticulum, and Golgi bodies similar to those found in other endocrine tissues as well as frequent PAS-positive granules. The juxtanuclear body of the light microscopists is identified with stacks of parallel lamellar elements of the endoplasmic rcticulum of the ergastoplasmic or granular type. Oxyphll cells are characterized by juxtanuclear bodies and by numerous mito- chondria found throughout their cytoplasm. Puzzling lamellar whorls are described in the cytoplasm of some oxyphil cells. The endothelium of parathyroid capillaries is extremely thin in some areas and contains numerous fenestrations as well as an extensive system of vesicles. The possible significance of these structures is discussed. The connective tissue elements found in the perivascular spaces of macaque parathyroid are described. INTRODUCTION Other contributions to the present concepts con cerning the human parathyroid can be found in the It is the purpose of the present paper to report some observations on the fine structure of the reports of Bergstrand (7), Morgan (34), Pappen- parathyroid gland employing the electron micro- heimer and Wilens (45), Castleman and Mallory (10), and Gilmour (20). -

Adrenal Gland Hormones
CHAPTER 8 Adrenal Gland Hormones Devra K. Dang, PharmD, BCPS, CDE, FNAP | Trinh Pham, PharmD, BCOP | Jennifer J. Lee, PharmD, BCPS, CDE LEARNING OBJECTIVES KEY TERMS AND DEFINITIONS After completing this chapter, you should be able to ACTH (adrenocorticotropic hormone) — a hormone produced 1. Identify the hormones produced by the adrenal glands by the pituitary gland that stimulates 2. Describe the functions of mineralocorticoids and glucocorticoids in the body the adrenal cortex to produce glucocorticoids, mineralocorticoids, 3. Recognize the signs and symptoms of adrenal insuffi ciency and androgens. PART 4. Describe the pharmacological treatment of patients with acute and chronic adrenal Addison ’ s disease — a disorder insuffi ciency in which the adrenal glands do not produce enough steroid hormones. 3 5. Recognize the signs and symptoms of Cushing ’ s syndrome and the result of too Adenoma — a benign much cortisol (noncancerous) tumor of glandular 6. Describe the pharmacologic and nonpharmacologic management of patients with origin. Cushing ’ s syndrome Adrenal insuffi ciency — a term 7. List management strategies for administration of glucocorticoid and mineralocorti- referring to a defi ciency in the levels of adrenal hormones. coid therapy to avoid development of adrenal disorders Aldosterone — the hormone produced by the adrenal glands that regulates the balance of sodium, he adrenal glands are an integral part of the endocrine system, secreting water, and potassium concentrations in the body. T hormones that act throughout the body to regulate functions and promote Corticotropin-releasing homeostasis. In addition to the neurotransmitters epinephrine and norepineph- hormone (CRH) — a hormone rine, the corticosteroids secreted by the adrenal glands are vital to a wide released by the hypothalamus that variety of physiological processes. -
Avian Adrenal Medulla: Cytomorphology and Function
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Publications of the IAS Fellows Volume 45(1-4):1-11, 2001 Acta Biologica Szegediensis http://www.sci.u-szeged.hu/ABS REVIEW ARTICLE Avian adrenal medulla: cytomorphology and function Asok Ghosh, Stephen W. Carmichael1*, Monisha Mukherjee Department of Zoology, University of Calcutta, Calcutta, India, 1Department of Anatomy, Mayo Clinic/Foundation, Rochester, Minnesota, USA ABSTRACT The purpose of this review is to explore the world literature on the avian adrenal KEY WORDS medulla from the last 20 years. Unlike the mammalian adrenal medulla, the adrenal gland in adrenal medulla birds has chromaffin cells mixed with cortical cells. Studies have investigated the ultrastructure birds (both transmission and scanning electron microscopy), biochemistry, and physiology (partic- morphology ularly interactions with other endocrine glands) of the avian adrenal medulla. Although function progress has been made, it is apparent that research on the avian adrenal medulla still lags behind work on the mammalian organ. Acta Biol Szeged 45(1-4):1-11 (2001) The adrenal glands of birds, like those in mammals, are in the adrenal medulla. This profound variation of medullary paired yellow- or orange-colored pear- or triangle-shaped E/NE ratio in birds suggests a distinct evolutionary pattern glands that are next to the kidneys. The intermingling nature (Ghosh 1977, 1980). The avian phylogeny used in this study of cortical and medullary components constitutes a major was essentially based on palaeontological evidences (Grego- characteristic of avian adrenal medulla (Vestergaard and ry 1957). We feel that our “claim” of hormonal taxonomy is Willeberg 1978).