Thyroid Gland Parathyroid Glands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fine Structure of the Parathyroid Gland*
The Fine Structure of the Parathyroid Gland* BY JERRY STEVEN TRIER, M.D. (From the Department of Anatomy, University of Washington School of Medicine, Seattle) PLATES 3 TO 10 (Received for publication, July 29, 1957) ABSTRACT The fine structure of the parathyroid of the macaque is described, and is cor- related with classical parathyroid cytology as seen in the light microscope. The two parenchymal cell types, the chief cells and the oxyphil cells, have been recognized in electron mierographs. The chief cells contain within their cyto- plasm mitochondria, endoplasmic reticulum, and Golgi bodies similar to those found in other endocrine tissues as well as frequent PAS-positive granules. The juxtanuclear body of the light microscopists is identified with stacks of parallel lamellar elements of the endoplasmic rcticulum of the ergastoplasmic or granular type. Oxyphll cells are characterized by juxtanuclear bodies and by numerous mito- chondria found throughout their cytoplasm. Puzzling lamellar whorls are described in the cytoplasm of some oxyphil cells. The endothelium of parathyroid capillaries is extremely thin in some areas and contains numerous fenestrations as well as an extensive system of vesicles. The possible significance of these structures is discussed. The connective tissue elements found in the perivascular spaces of macaque parathyroid are described. INTRODUCTION Other contributions to the present concepts con cerning the human parathyroid can be found in the It is the purpose of the present paper to report some observations on the fine structure of the reports of Bergstrand (7), Morgan (34), Pappen- parathyroid gland employing the electron micro- heimer and Wilens (45), Castleman and Mallory (10), and Gilmour (20). -

Thyroid & Parathyroid Glands
THYROID & PARATHYROID GLANDS Objectives: ◧ Editing file • Describe the histological structure of ◧ thyroid & parathyroid glands. Important • Identify and correlate between the different ◧ Doctor notes / Extra endocrine cells in thyroid gland and their functions. • Describe the functional structure of the parathyroid cells. 438 Histology Team Endocrine Block THYROID GLAND STROMA 1- Capsule: dense irregular collagenous C.T. 2- Septa (Interlobular septa) 3- Reticular fibers: Thin C.T. composed mostly of reticular fibers with rich capillary plexus surrounds each thyroid follicle. PARENCHYMA Are the structural and functional units of the thyroid gland. 1- Simple cuboidal epithelium: 2- Colloid: central colloid-filled lumen. a- Follicular (principal) cells b- Parafollicular cells (C cells) (Clear cells) - Pale-stained cells (Clear Cells). (Polygonal/pyramidal cells) N.B. Each follicle is - Simple cuboidal cells. - Found singly or in clusters in between the follicular cells. - Round nucleus with prominent nucleoli. surrounded by thin - Their apices do not reach the lumen of the follicle. L/M: - Basophilic cytoplasm. basal lamina. - Are larger than follicular cells (2-3 times).(larger but less in number) - Apical surface reaches the lumen of the (Acidophilic) - Only 0.1% of the epithelial cells. thyroid follicle. - Have round nucleus - Mitochondria. - RER. (synthesis of thyroglobulin) - Supranuclear Golgi Complex. - Mitochondria. E/M: - Numerous apically-located lysosomes. - RER (moderate). - Numerous dispersed small vesicles - Well-developed Golgi. - The vesicles contain newly formed thyroglobulin. - Numerous apical short microvilli. Function: Synthesis of thyroid hormones (T4 & T3). Secrete calcitonin. 438 Histology Team - Endocrine Block 2 PARATHYROID GLAND They are 4 glands on the posterior of thyroid gland. STROMA 1- Capsule: Each gland has its Thin capsule. -

Primary Hyperparathyroidism
Primary Hyperparathyroidism The parathyroid glands are 4 glands that reside within the thyroid glands: there are two parathyroid glands attached to each thyroid gland. The thyroid/parathyroid glands lie on either side of the trachea (wind pipe) about midway down the neck. The job of the parathyroid glands is to regulate calcium in the body. They secrete a hormone called parathyroid hormone (PTH), and this hormone draws calcium out of the bone where it is stored, as well as acting on the kidney and intestine to increase blood calcium. This is a normal process in response to fluctuations in blood calcium levels. The calcium in the blood is regulated by PTH (increases blood calcium) and calcitonin (a hormone secreted by the thyroid gland to decrease calcium) in order to keep it within a normal range. When the calcium gets too high or too low, symptoms arise. The most common symptoms of high calcium are increased thirst and urination. Other signs include: lethargy, weakness, diarrhea, inappetance, weight loss, tremors or stiffness. In about 50% of dogs with high calcium, urinary stones +/- urinary tract infection is present. Tumors (enlargement) of the parathyroid gland are typically not malignant, but they do over-secrete PTH. In 90% of cases only one of the four glands is enlarged. By one gland enlarging and over-secreting PTH, the three other glands typically shrink and temporarily stop functioning. Parathyroid tumors are suspected based on consistent blood work that shows elevated or normal PTH in the face of elevated calcium (the normal feedback mechanism would mean that when calcium is high, PTH is not needed and therefore is low). -

Parathyroid Disorders THOMAS C
Parathyroid Disorders THOMAS C. MICHELS, MD, MPH, Madigan Army Medical Center, Tacoma, Washington KEVIN M. KELLY, MD, MBA, Carl R. Darnall Army Community Hospital, Fort Hood, Texas Disorders of the parathyroid glands most commonly present with abnormalities of serum calcium. Patients with primary hyperparathyroidism, the most common cause of hypercalcemia in outpatients, are often asymptomatic or may have bone disease, nephrolithiasis, or neuromuscular symptoms. Patients with chronic kidney disease may develop secondary hyperparathyroidism with resultant chronic kidney disease-mineral and bone disorder. Hypo- parathyroidism most often occurs after neck surgery; it can also be caused by autoimmune destruction of the glands and other less common problems. Evaluation of patients with abnormal serum calcium levels includes a history and physical examination; repeat measurement of serum calcium level; and measurement of creatinine, magne- sium, vitamin D, and parathyroid hormone levels. The treatment for symptomatic primary hyperparathyroidism is parathyroidectomy. Management of asymptomatic primary hyperparathyroidism includes monitoring symptoms; serum calcium and creatinine levels; and bone mineral density. Patients with hypoparathyroidism require close monitoring and vitamin D (e.g., calcitriol) replacement. (Am Fam Physician. 2013;88(4):249-257. Copyright © 2013 American Academy of Family Physicians.) CME This clinical content he four parathyroid glands, located 84-amino acid peptide. PTH increases serum conforms to AAFP criteria posterior to the thyroid gland, reg- calcium levels through direct action on bone for continuing medical education (CME). See CME ulate calcium homeostasis through and the kidneys. It stimulates osteoclasts to Quiz on page 227. release of parathyroid hormone resorb bone and mobilize calcium into the T (PTH). Because most parathyroid disorders blood. -

Primary Hyperparathyroidism
Primary Hyperparathyroidism National Endocrine and Metabolic Diseases Information Service What is primary What are the parathyroid hyperparathyroidism? glands? Primary hyperparathyroidism is a disorder The parathyroid glands are four pea-sized U.S. Department of the parathyroid glands, also called glands located on or near the thyroid gland of Health and parathyroids. “Primary” means this disorder in the neck. Occasionally, a person is born Human Services originates in the parathyroid glands. In with one or more of the parathyroid glands primary hyperparathyroidism, one or more in another location. For example, a gland NATIONAL INSTITUTES of the parathyroid glands are overactive. may be embedded in the thyroid, in the OF HEALTH As a result, the gland releases too much thymus—an immune system organ located parathyroid hormone (PTH). The disorder in the chest—or elsewhere around this area. includes the problems that occur in the rest In most such cases, however, the parathyroid of the body as a result of too much PTH—for glands function normally. example, loss of calcium from bones. In the United States, about 100,000 people develop primary hyperparathyroidism each year.1 The disorder is diagnosed most often in people between age 50 and 60, and women are affected about three times as often as men.2 Secondary, or reactive, hyperparathyroidism can occur if a problem such as kidney failure causes the parathyroid glands to be overactive. Parathyroid glands Thyroid gland The parathyroid glands are located on or near the thyroid gland in the neck. 1Bilezikian JP. Primary hyperparathyroidism. In: DeGroot LJ, ed.; Arnold A, section editor. -
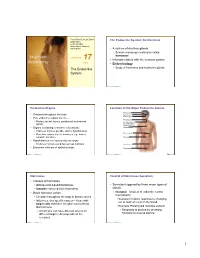
The Endocrine System
PowerPoint® Lecture Slides The Endocrine System: An Overview prepared by Leslie Hendon University of Alabama, Birmingham • A system of ductless glands • Secrete messenger molecules called hormones C H A P T E R 17 • Interacts closely with the nervous system Part 1 • Endocrinology The Endocrine • Study of hormones and endocrine glands System Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Endocrine Organs Location of the Major Endocrine Glands Pineal gland • Scattered throughout the body Hypothalamus Pituitary gland • Pure endocrine organs are the … Thyroid gland • Pituitary, pineal, thyroid, parathyroid, and adrenal Parathyroid glands glands (on dorsal aspect of thyroid gland) • Organs containing endocrine cells include: Thymus • Pancreas, thymus, gonads, and the hypothalamus Adrenal glands • Plus other organs secrete hormones (eg., kidney, stomach, intestine) Pancreas • Hypothalamus is a neuroendocrine organ • Produces hormones and has nervous functions Ovary (female) Endocrine cells are of epithelial origin • Testis (male) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 17.1 Hormones Control of Hormones Secretion • Classes of hormones • Amino acid–based hormones • Secretion triggered by three major types of • Steroids—derived from cholesterol stimuli: • Basic hormone action • Humoral—simplest of endocrine control mechanisms • Circulate throughout the body in blood vessels • Secretion in direct response to changing • Influences only specific tissues— those with ion or nutrient levels in the blood target cells that have receptor molecules for that hormone • Example: Parathyroid monitors calcium • A hormone can have different effects on • Responds to decline by secreting different target cells (depends on the hormone to reverse decline receptor) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. -

Preoperative and Intraoperative Methods of Parathyroid Gland Localization and the Diagnosis of Parathyroid Adenomas
molecules Review Preoperative and Intraoperative Methods of Parathyroid Gland Localization and the Diagnosis of Parathyroid Adenomas Jacek Baj 1,*, Robert Sitarz 1,2 , Marek Łokaj 2, Alicja Forma 1 , Marcin Czeczelewski 3 , Amr Maani 1 and Gabriella Garruti 4 1 Chair and Department of Anatomy, Medical University of Lublin, 20-950 Lublin, Poland; [email protected] (R.S.); [email protected] (A.F.); [email protected] (A.M.) 2 Department of Surgery, Center of Oncology of the Lublin Region St. Jana z Dukli, 20-090 Lublin, Poland; [email protected] 3 Chair and Department of Forensic Medicine, Medical University of Lublin, 20-950 Lublin, Poland; [email protected] 4 Section of Endocrinology, Andrology and Metabolic Diseases, Department of Emergency and Organ Transplantations, University of Bari “Aldo Moro” Medical School, 70124 Bari, Italy; [email protected] * Correspondence: [email protected]; Tel.: +48-662-094-014 Received: 24 February 2020; Accepted: 8 April 2020; Published: 9 April 2020 Abstract: Accurate pre-operative determination of parathyroid glands localization is critical in the selection of minimally invasive parathyroidectomy as a surgical treatment approach in patients with primary hyperparathyroidism (PHPT). Its importance cannot be overemphasized as it helps to minimize the harmful side effects associated with damage to the parathyroid glands such as in hypocalcemia, severe hemorrhage or recurrent laryngeal nerve dysfunction. Preoperative and intraoperative methods decrease the incidence of mistakenly -

Anatomy of Endocrine System
Anatomy of Endocrine system Introduction, Pituitary gland and Thyroid gland Prepared by Dr. Payal Jain Endocrine System I. Introduction A. Considered to be part of animals communication system 1. Nervous system uses physical structures for communication 2. Endocrine system uses body fluids to transport messages (hormones) II. Hormones A. Classically, hormones are defined as chemical substances produced by ductless glands and secreted into the blood supply to affect a tissue distant from the gland, but now it is understood that hormones can be produced by single cells as well. 1. epicrine a. hormones pass through gap junctions of adjacent cells without entering extracellular fluid 2. paracrine a. hormones diffuse through interstitial fluid (e.g. prostaglandins) 3. endocrine a. hormones are delivered via the bloodstream (e.g. growth hormone Different endocrine glands with cell Organ Division arrangement Cell arrangement/morphology Hormone Hypophysis Adenohypophysis Pars distalis Cells in cords around large-bore capillaries: Acidophils Growth hormone, prolactin Basophils ACTH, TSH, FSH, LH Pars intermedia Mostly basophilic cells around ACTH, POMC cystic cavities Pars tuberalis Narrow sleeve of basophilc cells LH around infundibulum Neurohypophysis Pars nervosa Nerve fibers and supporting cells Oxytocin and (pituicytes) vasopressin (produced in hypothalamus) Infundibulum Nerve fibers (traveling from hypothalamus to pars nervosa) Pancreas Islet of Langerhans Irregularly arranged cells with Insulin, glucagon many capillaries Follicles: Simple -
![Endocrine Glands [PDF]](https://docslib.b-cdn.net/cover/5879/endocrine-glands-pdf-3555879.webp)
Endocrine Glands [PDF]
Histology of Skin and Endocrine glands Skin and Endocrine glands • Skin • Thyroid • Parathyroid gland • Adrenal gland • Pituitary gland • Pineal gland Skin • Layers of skin • Epidermis • Five layers • Dermis • Two layers Junction • Dermal papilla • Epidermal peg (rete pegs) Skin…. • Epidermis - 1.Stratum basale • Single layer of columnar cells 2.Stratum spinosum • Several layers of polyhedral cells, spine like process, tonofilament 3.Stratum granulosum • Keratohyline granules 4.Stratum lucidum • Homogeneous keratin, fusiform cells 5.Stratum corneum-non nucleated keratinized dead cells Skin…… • Cells of epidermis -Keratinocytes- 90%,able to keratinization -Cells of Langherhans- present in st.spinosum, clear cytoplasmic process, antigen producing cell -Melamocytes-pigmented cell in basal layer, many cytoplasmic process. Produce Melanin -Merkel cells- sensory cell Skin….. Dermis • Papillary layer • Tactile papilla • Vascular papilla • Collagen fibre • Reticular layer Collagen fibre • Sweat glands • Sebaceous glands • Hairs Skin…… • Thick skin • Thin skin Thyroid gland 1.Capsule 2.Parenchyma • thyroid follicle -Structural & functional unite -Epithelium-simple cuboidal cells (follicular cells), synthesis thyroxin hormone -cell size varies with activeness -Lumen of thyroid follicle filled with colloid -Parafollicular cells (“C” Cells) Present at margin or inter follicular space, Calcitonin 3.Stroma- connective tissue, septa, blood vessels Parathyroid gland • Chief cells • Polygonal shape, round nucleus, synthesis Parathormone • Oxyphill cells- -

The Normal Thyroid Gland
1 THE NORMAL THYROID GLAND EMBRYOLOGY before lumen formation and colloid secretion The thyroid anlage appears as a bilobate ve- can be detected (4,5). sicular structure at the foramen cecum of the It has recently become clear that thyroid gland tongue. It then descends as a component of the organogenesis and the differentiation of follicular thyroglossal duct to reach its definitive position in cells are directed by the concerted action of a series the neck (fig. -). After involution of the thyro- of transcription factors, while thyroid-stimulating glossal duct, the thyroid anlage begins to expand hormone (TSH) influences thyroid differentiation laterally to form the thyroid lobes (–3). only after the anatomic outline of the gland is well Microscopically, the initially solid thyroid established. The most important of these tran- anlage begins to form cords and plates of fol- scription factors are thyroid transcription factor licular cells during the 9th gestational week. A (TTF)- (Nkx2-), TTF-2 (Foxe), PAX8, and Hhex small lumen appears within the follicles by the (6). Although these factors are also expressed and 0th week, with colloid secretion becoming influence differentiation in other developing tis- evident by the 2th week. By the 4th week, sues, all four are coexpressed only in the thyroid the gland already consists of well-developed anlage (6). Since they regulate the expression follicles lined by follicular cells and containing of thyroid-specific genes (e.g., those responsible thyroglobulin-positive colloid in their lumens for the production of thyroid peroxidase and (figs. -2–-3). Labeled amino acid studies have thyroglobulin) they are important not only for shown that thyroglobulin synthesis begins at a organogenesis but for the functional differen- very early stage, when the thyroid gland is still tiation of the gland in later stages of prenatal a solid mass at the base of the tongue, and long development and postnatally (6,7). -

What Is Endocrine Surgery?
What is Endocrine Surgery? Endocrine Surgery is the discipline of surgical management of endocrine disorders, including the understanding of disease process, technical mastery and comprehensive care of surgical endocrine diseases of the neck and abdomen. Thyroid The thyroid gland is a butterfly shaped gland in the center of the neck. The thyroid gland produces the thyroid hormones T3 (triiodothyronine) and T4 (thyroxine). These hormones regulate the growth and function of many systems of the body and they set the pace of the metabolism. Hypothyroidism is when the thyroid under produces hormones. Hashimoto’s thyroiditis is an auto-immune disease in which the immune system attacks the thyroid gland resulting in hypothyroidism. Hyperthyroidism is overproduction of thyroid hormone. Graves’ disease is also an autoimmune disease however in this case the result is excessive thyroid hormone levels and hyperthyroidism. Goiter is a term for enlargement of the thyroid gland. The enlargement can be diffuse, due to one nodule or due to multiple nodules. Nodules are growths within the thyroid gland. Nodules can be benign or malignant. Nodules are evaluated with a neck ultrasound. Nodules greater than 1cm or with suspicious features should be sampled with a fine needle biopsy to rule out a thyroid cancer. Larger nodules can exert pressure on local structures such as the trachea, esophagus, and nerve to the voice resulting in trouble breathing, swallowing or speaking. Thyroid surgery is indicated when nodules cause compressive symptoms, when nodules are cancerous and when nodules are suspicious on fine needle aspiration. Thyroid cancer is cancer originating in the thyroid gland. There are 4 types of thyroid cancer: papillary carcinoma, follicular carcinoma, medullary carcinoma and anaplastic thyroid cancer. -

Rodriguez, Elvira Fernandez and Jose M. Valdivielso Eva Parisi, Yolanda Almadén, Mercé Ibarz, Sara Panizo, Anna Cardús, Maria
Eva Parisi, Yolanda Almadén, Mercé Ibarz, Sara Panizo, Anna Cardús, Mariano Rodriguez, Elvira Fernandez and Jose M. Valdivielso Am J Physiol Renal Physiol 296:1291-1296, 2009. First published Apr 8, 2009; doi:10.1152/ajprenal.90557.2008 You might find this additional information useful... This article cites 25 articles, 12 of which you can access free at: http://ajprenal.physiology.org/cgi/content/full/296/6/F1291#BIBL Updated information and services including high-resolution figures, can be found at: http://ajprenal.physiology.org/cgi/content/full/296/6/F1291 Additional material and information about AJP - Renal Physiology can be found at: http://www.the-aps.org/publications/ajprenal This information is current as of June 2, 2009 . Downloaded from ajprenal.physiology.org on June 2, 2009 AJP - Renal Physiology publishes original manuscripts on a broad range of subjects relating to the kidney, urinary tract, and their respective cells and vasculature, as well as to the control of body fluid volume and composition. It is published 12 times a year (monthly) by the American Physiological Society, 9650 Rockville Pike, Bethesda MD 20814-3991. Copyright © 2005 by the American Physiological Society. ISSN: 0363-6127, ESSN: 1522-1466. Visit our website at http://www.the-aps.org/. Am J Physiol Renal Physiol 296: F1291–F1296, 2009. First published April 8, 2009; doi:10.1152/ajprenal.90557.2008. N-methyl-D-aspartate receptors are expressed in rat parathyroid gland and regulate PTH secretion Eva Parisi,1 Yolanda Almade´n,3 Merce´ Ibarz,4 Sara Panizo,1 Anna Cardu´s,1 Mariano Rodriguez,3 Elvira Fernandez,2* and Jose M.