The Fine Structure of the Parathyroid Gland*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2013/096741 A2 27 June 2013 (27.06.2013) P CT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/096741 A2 27 June 2013 (27.06.2013) P CT (51) International Patent Classification: (74) Agents: GEORGE, Nikolaos C. et al; Jones Day, 222 A61K 35/12 (2006.01) East 41st Street, New York, NY 10017-6702 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/US20 12/07 1192 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (22) Date: International Filing BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 2 1 December 2012 (21 .12.2012) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (26) Publication Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (30) Priority Data: NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, 61/579,942 23 December 201 1 (23. 12.201 1) US RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, 61/592,350 30 January 2012 (30.01.2012) US TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, 61/696,527 4 September 2012 (04.09.2012) us ZM, ZW. (71) Applicant: ANTHROGENESIS CORPORATION (84) Designated States (unless otherwise indicated, for every [US/US]; 33 Technology Drive, Warren, NJ 07059 (US). -

Thyroid Gland Parathyroid Glands
Human Physiology Course Thyroid Gland Parathyroid Glands Assoc. Prof. Mária Pallayová, MD, PhD [email protected] Department of Human Physiology, UPJŠ LF April 13, 2020 (10th week – Summer Semester 2019/2020) Hormones and Functions Thyroid gland Parathyroids Thymus Adrenal glands Endocrine pancreas Ovaries, Testes Pineal Pituitary Hypothalamus-Pituitary Axis GIT, adipose tissue, brain, heart, kidney, ... Hormones and Functions Thyroid gland Parathyroids Thymus Adrenal glands Endocrine pancreas Ovaries, Testes Pineal Pituitary Hypothalamus-Pituitary Axis GIT, adipose tissue, brain, heart, kidney, ... Lecture Outline Functional anatomy of the thyroid gland Synthesis, secretion, and metabolism of the thyroid hormones The mechanism of thyroid action Role of the thyroid hormones in development, growth, and metabolism Thyroid hormone deficiency and excess in adults Physiology of the parathyroids Functional Anatomy of the Thyroid Gland two lobes + isthmus (just below the cricoid cartilage) attached to the trachea by connective tissue A normal THGL in a healthy adult weighs about 15-20 g. Functional Anatomy of the Thyroid Gland arterial blood supply: from a superior and an inferior thyroid a., which arise from the external carotid and subclavian a., respectively. venous blood supply: a series of thyroid veins drain into the ext. jugular and innominate vv. ( a rich blood supply to the thyroid gland w/ a higher rate of blood flow per gram than even that of the kidneys). innervation: adrenergic innervation from the cervical ganglia; cholinergic innervation from the n. vagus (regulation of vasomotor function to increase the delivery of TSH, iodide, and metabolic substrates to the THGL). Functional Anatomy of the Thyroid Gland The colloid (a thick, gel-like substance) is a solution composed primarily of thyroglobulin (10-25% the high viscosity), a large protein that is a storage form of the thyroid hormones. -

WO 2015/168656 A2 5 November 2015 (05.11.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/168656 A2 5 November 2015 (05.11.2015) P O P C T (51) International Patent Classification: (72) Inventors: HSIAO, Sonny; 1985 Pleasant Valley Avenue, A61K 48/00 (2006.01) Apartment 7, Oakland, CA 9461 1 (US). LIU, Cheng; 24 N Hill Court, Oakland, CA 94618 (US). LIU, Hong; 5573 (21) International Application Number: Woodview Drive, El Sobrante, CA 94803 (US). PCT/US20 15/02895 1 (74) Agents: GIERING, Jeffery, C. et al; Wilson Sonsini (22) International Filing Date: Goodrich & Rosati, 650 Page Mill Road, Palo Alto, CA 1 May 2015 (01 .05.2015) 94304-1050 (US). (25) Filing Language: English (81) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (30) Priority Data: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 61/988,070 2 May 2014 (02.05.2014) US DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (71) Applicant: ADHEREN INCORPORATED [US/US]; HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 1026 Rispin Drive, Berkeley, CA 94705 (US). KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (72) Inventors; and PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (71) Applicants : TWITE, Amy, A. -

Nomenclatore Per L'anatomia Patologica Italiana Arrigo Bondi
NAP Nomenclatore per l’Anatomia Patologica Italiana Versione 1.9 Arrigo Bondi Bologna, 2016 NAP v. 1.9, pag 2 Arrigo Bondi * NAP - Nomenclatore per l’Anatomia Patologica Italiana Versione 1.9 * Componente Direttivo Nazionale SIAPEC-IAP Società Italiana di Anatomia Patologica e Citodiagnostica International Academy of Pathology, Italian Division NAP – Depositato presso S.I.A.E. Registrazione n. 2012001925 Distribuito da Palermo, 1 Marzo 2016 NAP v. 1.9, pag 3 Sommario Le novità della versione 1.9 ............................................................................................................... 4 Cosa è cambiato rispetto alla versione 1.8 ........................................................................................... 4 I Nomenclatori della Medicina. ........................................................................................................ 5 ICD, SNOMED ed altri sistemi per la codifica delle diagnosi. ........................................................... 5 Codifica medica ........................................................................................................................... 5 Storia della codifica in medicina .................................................................................................. 5 Lo SNOMED ............................................................................................................................... 6 Un Nomenclatore per l’Anatomia Patologica Italiana ................................................................. 6 Il NAP ................................................................................................................................................. -

Thyroid & Parathyroid Glands
THYROID & PARATHYROID GLANDS Objectives: ◧ Editing file • Describe the histological structure of ◧ thyroid & parathyroid glands. Important • Identify and correlate between the different ◧ Doctor notes / Extra endocrine cells in thyroid gland and their functions. • Describe the functional structure of the parathyroid cells. 438 Histology Team Endocrine Block THYROID GLAND STROMA 1- Capsule: dense irregular collagenous C.T. 2- Septa (Interlobular septa) 3- Reticular fibers: Thin C.T. composed mostly of reticular fibers with rich capillary plexus surrounds each thyroid follicle. PARENCHYMA Are the structural and functional units of the thyroid gland. 1- Simple cuboidal epithelium: 2- Colloid: central colloid-filled lumen. a- Follicular (principal) cells b- Parafollicular cells (C cells) (Clear cells) - Pale-stained cells (Clear Cells). (Polygonal/pyramidal cells) N.B. Each follicle is - Simple cuboidal cells. - Found singly or in clusters in between the follicular cells. - Round nucleus with prominent nucleoli. surrounded by thin - Their apices do not reach the lumen of the follicle. L/M: - Basophilic cytoplasm. basal lamina. - Are larger than follicular cells (2-3 times).(larger but less in number) - Apical surface reaches the lumen of the (Acidophilic) - Only 0.1% of the epithelial cells. thyroid follicle. - Have round nucleus - Mitochondria. - RER. (synthesis of thyroglobulin) - Supranuclear Golgi Complex. - Mitochondria. E/M: - Numerous apically-located lysosomes. - RER (moderate). - Numerous dispersed small vesicles - Well-developed Golgi. - The vesicles contain newly formed thyroglobulin. - Numerous apical short microvilli. Function: Synthesis of thyroid hormones (T4 & T3). Secrete calcitonin. 438 Histology Team - Endocrine Block 2 PARATHYROID GLAND They are 4 glands on the posterior of thyroid gland. STROMA 1- Capsule: Each gland has its Thin capsule. -

Primary Hyperparathyroidism
Primary Hyperparathyroidism The parathyroid glands are 4 glands that reside within the thyroid glands: there are two parathyroid glands attached to each thyroid gland. The thyroid/parathyroid glands lie on either side of the trachea (wind pipe) about midway down the neck. The job of the parathyroid glands is to regulate calcium in the body. They secrete a hormone called parathyroid hormone (PTH), and this hormone draws calcium out of the bone where it is stored, as well as acting on the kidney and intestine to increase blood calcium. This is a normal process in response to fluctuations in blood calcium levels. The calcium in the blood is regulated by PTH (increases blood calcium) and calcitonin (a hormone secreted by the thyroid gland to decrease calcium) in order to keep it within a normal range. When the calcium gets too high or too low, symptoms arise. The most common symptoms of high calcium are increased thirst and urination. Other signs include: lethargy, weakness, diarrhea, inappetance, weight loss, tremors or stiffness. In about 50% of dogs with high calcium, urinary stones +/- urinary tract infection is present. Tumors (enlargement) of the parathyroid gland are typically not malignant, but they do over-secrete PTH. In 90% of cases only one of the four glands is enlarged. By one gland enlarging and over-secreting PTH, the three other glands typically shrink and temporarily stop functioning. Parathyroid tumors are suspected based on consistent blood work that shows elevated or normal PTH in the face of elevated calcium (the normal feedback mechanism would mean that when calcium is high, PTH is not needed and therefore is low). -

Wednesday Slide Conference 2008-2009
PROCEEDINGS DEPARTMENT OF VETERINARY PATHOLOGY WEDNESDAY SLIDE CONFERENCE 2008-2009 ARMED FORCES INSTITUTE OF PATHOLOGY WASHINGTON, D.C. 20306-6000 2009 ML2009 Armed Forces Institute of Pathology Department of Veterinary Pathology WEDNESDAY SLIDE CONFERENCE 2008-2009 100 Cases 100 Histopathology Slides 249 Images PROCEEDINGS PREPARED BY: Todd Bell, DVM Chief Editor: Todd O. Johnson, DVM, Diplomate ACVP Copy Editor: Sean Hahn Layout and Copy Editor: Fran Card WSC Online Management and Design Scott Shaffer ARMED FORCES INSTITUTE OF PATHOLOGY Washington, D.C. 20306-6000 2009 ML2009 i PREFACE The Armed Forces Institute of Pathology, Department of Veterinary Pathology has conducted a weekly slide conference during the resident training year since 12 November 1953. This ever- changing educational endeavor has evolved into the annual Wednesday Slide Conference program in which cases are presented on 25 Wednesdays throughout the academic year and distributed to 135 contributing military and civilian institutions from around the world. Many of these institutions provide structured veterinary pathology resident training programs. During the course of the training year, histopathology slides, digital images, and histories from selected cases are distributed to the participating institutions and to the Department of Veterinary Pathology at the AFIP. Following the conferences, the case diagnoses, comments, and reference listings are posted online to all participants. This study set has been assembled in an effort to make Wednesday Slide Conference materials available to a wider circle of interested pathologists and scientists, and to further the education of veterinary pathologists and residents-in-training. The number of histopathology slides that can be reproduced from smaller lesions requires us to limit the number of participating institutions. -

Parathyroid Disorders THOMAS C
Parathyroid Disorders THOMAS C. MICHELS, MD, MPH, Madigan Army Medical Center, Tacoma, Washington KEVIN M. KELLY, MD, MBA, Carl R. Darnall Army Community Hospital, Fort Hood, Texas Disorders of the parathyroid glands most commonly present with abnormalities of serum calcium. Patients with primary hyperparathyroidism, the most common cause of hypercalcemia in outpatients, are often asymptomatic or may have bone disease, nephrolithiasis, or neuromuscular symptoms. Patients with chronic kidney disease may develop secondary hyperparathyroidism with resultant chronic kidney disease-mineral and bone disorder. Hypo- parathyroidism most often occurs after neck surgery; it can also be caused by autoimmune destruction of the glands and other less common problems. Evaluation of patients with abnormal serum calcium levels includes a history and physical examination; repeat measurement of serum calcium level; and measurement of creatinine, magne- sium, vitamin D, and parathyroid hormone levels. The treatment for symptomatic primary hyperparathyroidism is parathyroidectomy. Management of asymptomatic primary hyperparathyroidism includes monitoring symptoms; serum calcium and creatinine levels; and bone mineral density. Patients with hypoparathyroidism require close monitoring and vitamin D (e.g., calcitriol) replacement. (Am Fam Physician. 2013;88(4):249-257. Copyright © 2013 American Academy of Family Physicians.) CME This clinical content he four parathyroid glands, located 84-amino acid peptide. PTH increases serum conforms to AAFP criteria posterior to the thyroid gland, reg- calcium levels through direct action on bone for continuing medical education (CME). See CME ulate calcium homeostasis through and the kidneys. It stimulates osteoclasts to Quiz on page 227. release of parathyroid hormone resorb bone and mobilize calcium into the T (PTH). Because most parathyroid disorders blood. -

Differentiation of Human Parathyroid Cells in Culture
417 Differentiation of human parathyroid cells in culture W Liu, P Ridefelt1, G Åkerström and P Hellman Department of Surgery, University Hospital, Uppsala, Sweden 1Clinical Chemistry, University Hospital, Uppsala, Sweden (Requests for offprints should be addressed to P Hellman, Department of Surgery, University Hospital, SE-751 85 Uppsala, Sweden; Email: [email protected]) Abstract Continuous culture of parathyroid cells has proven diffi- histochemistry for proliferating cell nuclear antigen and cult, regardless from which species the cells are derived. In cell counting. Signs of differentiation were present as the the present study, we have used a defined serum-free low set-points, defined as the external calcium concentration at 2+ calcium containing medium to culture human parathyroid which half-maximal stimulation of [Ca ]i (set-pointc), or cells obtained from patients with parathyroid adenomas half-maximal inhibition of PTH release (set-pointp) occur, due to primary hyperparathyroidism. No fibroblast over- were higher in not proliferating compared with prolifer- growth occurred, and the human parathyroid chief cells ating cells in P0. Inhibition of cell proliferation was proliferated until confluent. After the first passage the cells accompanied by signs of left-shifted set-points, indicating ceased to proliferate, but still retained their functional a link between proliferation and differentiation. capacity up to 60 days, demonstrated by Ca2+-sensitive The results demonstrate that human parathyroid chief changes in the release of parathyroid hormone (PTH) and cells cultured in a defined serum-free medium can be kept 2+ ff as adequate cytoplasmic calcium ([Ca ]i) responses to viable for a considerable time, and that signs of di er- changes in ambient calcium as measured by micro- entiation occur after proliferation has ceased. -

Primary Hyperparathyroidism
Primary Hyperparathyroidism National Endocrine and Metabolic Diseases Information Service What is primary What are the parathyroid hyperparathyroidism? glands? Primary hyperparathyroidism is a disorder The parathyroid glands are four pea-sized U.S. Department of the parathyroid glands, also called glands located on or near the thyroid gland of Health and parathyroids. “Primary” means this disorder in the neck. Occasionally, a person is born Human Services originates in the parathyroid glands. In with one or more of the parathyroid glands primary hyperparathyroidism, one or more in another location. For example, a gland NATIONAL INSTITUTES of the parathyroid glands are overactive. may be embedded in the thyroid, in the OF HEALTH As a result, the gland releases too much thymus—an immune system organ located parathyroid hormone (PTH). The disorder in the chest—or elsewhere around this area. includes the problems that occur in the rest In most such cases, however, the parathyroid of the body as a result of too much PTH—for glands function normally. example, loss of calcium from bones. In the United States, about 100,000 people develop primary hyperparathyroidism each year.1 The disorder is diagnosed most often in people between age 50 and 60, and women are affected about three times as often as men.2 Secondary, or reactive, hyperparathyroidism can occur if a problem such as kidney failure causes the parathyroid glands to be overactive. Parathyroid glands Thyroid gland The parathyroid glands are located on or near the thyroid gland in the neck. 1Bilezikian JP. Primary hyperparathyroidism. In: DeGroot LJ, ed.; Arnold A, section editor. -
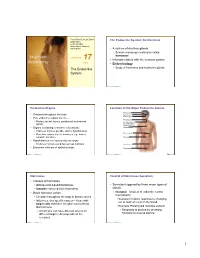
The Endocrine System
PowerPoint® Lecture Slides The Endocrine System: An Overview prepared by Leslie Hendon University of Alabama, Birmingham • A system of ductless glands • Secrete messenger molecules called hormones C H A P T E R 17 • Interacts closely with the nervous system Part 1 • Endocrinology The Endocrine • Study of hormones and endocrine glands System Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Endocrine Organs Location of the Major Endocrine Glands Pineal gland • Scattered throughout the body Hypothalamus Pituitary gland • Pure endocrine organs are the … Thyroid gland • Pituitary, pineal, thyroid, parathyroid, and adrenal Parathyroid glands glands (on dorsal aspect of thyroid gland) • Organs containing endocrine cells include: Thymus • Pancreas, thymus, gonads, and the hypothalamus Adrenal glands • Plus other organs secrete hormones (eg., kidney, stomach, intestine) Pancreas • Hypothalamus is a neuroendocrine organ • Produces hormones and has nervous functions Ovary (female) Endocrine cells are of epithelial origin • Testis (male) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 17.1 Hormones Control of Hormones Secretion • Classes of hormones • Amino acid–based hormones • Secretion triggered by three major types of • Steroids—derived from cholesterol stimuli: • Basic hormone action • Humoral—simplest of endocrine control mechanisms • Circulate throughout the body in blood vessels • Secretion in direct response to changing • Influences only specific tissues— those with ion or nutrient levels in the blood target cells that have receptor molecules for that hormone • Example: Parathyroid monitors calcium • A hormone can have different effects on • Responds to decline by secreting different target cells (depends on the hormone to reverse decline receptor) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. -

Preoperative and Intraoperative Methods of Parathyroid Gland Localization and the Diagnosis of Parathyroid Adenomas
molecules Review Preoperative and Intraoperative Methods of Parathyroid Gland Localization and the Diagnosis of Parathyroid Adenomas Jacek Baj 1,*, Robert Sitarz 1,2 , Marek Łokaj 2, Alicja Forma 1 , Marcin Czeczelewski 3 , Amr Maani 1 and Gabriella Garruti 4 1 Chair and Department of Anatomy, Medical University of Lublin, 20-950 Lublin, Poland; [email protected] (R.S.); [email protected] (A.F.); [email protected] (A.M.) 2 Department of Surgery, Center of Oncology of the Lublin Region St. Jana z Dukli, 20-090 Lublin, Poland; [email protected] 3 Chair and Department of Forensic Medicine, Medical University of Lublin, 20-950 Lublin, Poland; [email protected] 4 Section of Endocrinology, Andrology and Metabolic Diseases, Department of Emergency and Organ Transplantations, University of Bari “Aldo Moro” Medical School, 70124 Bari, Italy; [email protected] * Correspondence: [email protected]; Tel.: +48-662-094-014 Received: 24 February 2020; Accepted: 8 April 2020; Published: 9 April 2020 Abstract: Accurate pre-operative determination of parathyroid glands localization is critical in the selection of minimally invasive parathyroidectomy as a surgical treatment approach in patients with primary hyperparathyroidism (PHPT). Its importance cannot be overemphasized as it helps to minimize the harmful side effects associated with damage to the parathyroid glands such as in hypocalcemia, severe hemorrhage or recurrent laryngeal nerve dysfunction. Preoperative and intraoperative methods decrease the incidence of mistakenly