The Adrenal Capsule Is a Signaling Center Controlling Cell Renewal and Zonation Through Rspo3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hedgehog Signaling Is Evolutionarily Conserved Cilium-Independent
Downloaded from genesdev.cshlp.org on August 14, 2009 - Published by Cold Spring Harbor Laboratory Press Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved Miao-Hsueh Chen, Christopher W. Wilson, Ya-Jun Li, et al. Genes Dev. 2009 23: 1910-1928 Access the most recent version at doi:10.1101/gad.1794109 Supplemental http://genesdev.cshlp.org/content/suppl/2009/07/23/23.16.1910.DC1.html Material References This article cites 97 articles, 47 of which can be accessed free at: http://genesdev.cshlp.org/content/23/16/1910.full.html#ref-list-1 Email alerting Receive free email alerts when new articles cite this article - sign up in the box at the service top right corner of the article or click here To subscribe to Genes & Development go to: http://genesdev.cshlp.org/subscriptions Copyright © 2009 by Cold Spring Harbor Laboratory Press Downloaded from genesdev.cshlp.org on August 14, 2009 - Published by Cold Spring Harbor Laboratory Press Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved Miao-Hsueh Chen,1,3 Christopher W. Wilson,1,3 Ya-Jun Li,1 Kelvin King Lo Law,2 Chi-Sheng Lu,1 Rhodora Gacayan,1 Xiaoyun Zhang,2 Chi-chung Hui,2 and Pao-Tien Chuang1,4 1Cardiovascular Research Institute, University of California at San Francisco, San Francisco, California 94158, USA; 2Program in Developmental and Stem Cell Biology, The Hospital for Sick Children, and Department of Molecular Genetics, University of Toronto, Toronto, Ontario M5G 1L7, Canada A central question in Hedgehog (Hh) signaling is how evolutionarily conserved components of the pathway might use the primary cilium in mammals but not fly. -

GLI1-Amplifications Expand the Spectrum of Soft Tissue Neoplasms Defined by GLI1 Gene Fusions
Modern Pathology (2019) 32:1617–1626 https://doi.org/10.1038/s41379-019-0293-x ARTICLE GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions 1 1 1 2 3 Narasimhan P. Agaram ● Lei Zhang ● Yun-Shao Sung ● Samuel Singer ● Todd Stevens ● 3 4 5 6 6 Carlos N. Prieto-Granada ● Justin A. Bishop ● Benjamin A. Wood ● David Swanson ● Brendan C. Dickson ● Cristina R. Antonescu1 Received: 18 February 2019 / Revised: 29 April 2019 / Accepted: 1 May 2019 / Published online: 12 June 2019 © United States & Canadian Academy of Pathology 2019 Abstract GLI1 fusions involving ACTB, MALAT1, and PTCH1 genes have been recently reported in a subset of malignant soft tissue tumors with characteristic monomorphic nested epithelioid morphology and frequent S100 positivity. However, we encountered a group of morphologically similar soft tissue tumors lacking the canonical GLI1 gene fusions and sought to investigate their genetic abnormalities. A combined approach including RNA sequencing, targeted exome sequencing and FISH methodologies were used to identify potential novel genetic abnormalities. Ten patients (five females, five males) with – fi 1234567890();,: 1234567890();,: an age range of 4 65 years (median 32.5) were identi ed. Tumors were located in the soft tissues of the limbs, trunk and head and neck, with one each in the tongue and lung. Histologically, tumors revealed ovoid to epithelioid cells arranged in a distinctive nested-trabecular pattern, separated by thin septa and a delicate vascular network. Two cases showed areas of increased nuclear pleomorphism and focal fascicular spindle cell growth. Four tumors showed a high mitotic count (≥15/10 HPFs), with necrosis seen in three of them. -

Non-Canonical Activation of Hedgehog in Prostate Cancer Cells Mediated by the Interaction of Transcriptionally Active Androgen Receptor Proteins with Gli3
Oncogene (2018) 37:2313–2325 https://doi.org/10.1038/s41388-017-0098-7 ARTICLE Non-canonical activation of hedgehog in prostate cancer cells mediated by the interaction of transcriptionally active androgen receptor proteins with Gli3 1 1,2 2 1 1 1 2,3 Na Li ● Sarah Truong ● Mannan Nouri ● Jackson Moore ● Nader Al Nakouzi ● Amy Anne Lubik ● Ralph Buttyan Received: 19 July 2017 / Revised: 18 October 2017 / Accepted: 29 November 2017 / Published online: 12 February 2018 © The Author(s) 2018. This article is published with open access Abstract Hedgehog (Hh) is an oncogenic signaling pathway that regulates the activity of Gli transcription factors. Canonical Hh is a Smoothened-(Smo-) driven process that alters the post-translational processing of Gli2/Gli3 proteins. Though evidence supports a role for Gli action in prostate cancer (PCa) cell growth and progression, there is little indication that Smo is involved. Here we describe a non-canonical means for activation of Gli transcription in PCa cells mediated by the binding of transcriptionally-active androgen receptors (ARs) to Gli3. Androgens stimulated reporter expression from a Gli-dependent promoter in a variety of AR + PCa cells and this activity was suppressed by an anti-androgen, Enz, or by AR knockdown. 1234567890();,: Androgens also upregulated expression of endogenous Gli-dependent genes. This activity was associated with increased intranuclear binding of Gli3 to AR that was antagonized by Enz. Fine mapping of the AR binding domain on Gli2 showed that AR recognizes the Gli protein processing domain (PPD) in the C-terminus. Mutations in the arginine-/serine repeat elements of the Gli2 PPD involved in phosphorylation and ubiquitinylation blocked the binding to AR. -

Histogenesis of Suprarenal Glands at Different Gestational Age Groups
ORIGINAL ARTICLE ASIAN JOURNAL OF MEDICAL SCIENCES Histogenesis of suprarenal glands at different gestational age groups Ravindra Kumar Boddeti1, Subhadra Devi Velichety2 1Lecturer, 2Professor and Head, Department of Anatomy, Sri Padmavathi Medical College for Women, Sri Venkateswara Institute of Medical Sciences, SVIMS University, Tirupathi, Andhra Pradesh, India Submitted: 22-02-2019 Revised: 10-03-2019 Published: 01-05-2019 ABSTRACT Background: The human foetal suprarenal gland is structurally variant from its adult Access this article online counterpart. The most distinctive features of human foetal suprarenal gland and histologically Website: unique foetal zone, was described first by Elliott and Armour in 1911. After the first trimester, the centrally located foetal zone accounts for most of the foetal adrenal mass. The outer zone http://nepjol.info/index.php/AJMS of the foetal suprarenal gland is called the “definitive zone or neo cortex”; this zone likely DOI: 10.3126/ajms.v10i3.22820 gives rise to the adult adrenal glomerulosa. A third zone called “transitional zone”, lies just E-ISSN: 2091-0576 2467-9100 between the neocortex and foetal zone and is believed to develop into the zona fasciculata. P-ISSN: Aims and Objectives: The current study was designed to study the histogenesis of suprarenal glands at different gestational age groups. Materials and Methods: Twenty-eight formalin preserved dead embryos and foetuses of both sexes, were obtained from the Govt. Maternity Hospital & S.V.Medical College, Tirupati, Andhra Pradesh, India. Specimens were grouped according to their gestational age groups (A,B,C,D) A= 0-12 weeks, B= 13-24 weeks, C= 25-36 weeks and D= more than 36 weeks of gestation. -

Adrenal Gland Hormones
CHAPTER 8 Adrenal Gland Hormones Devra K. Dang, PharmD, BCPS, CDE, FNAP | Trinh Pham, PharmD, BCOP | Jennifer J. Lee, PharmD, BCPS, CDE LEARNING OBJECTIVES KEY TERMS AND DEFINITIONS After completing this chapter, you should be able to ACTH (adrenocorticotropic hormone) — a hormone produced 1. Identify the hormones produced by the adrenal glands by the pituitary gland that stimulates 2. Describe the functions of mineralocorticoids and glucocorticoids in the body the adrenal cortex to produce glucocorticoids, mineralocorticoids, 3. Recognize the signs and symptoms of adrenal insuffi ciency and androgens. PART 4. Describe the pharmacological treatment of patients with acute and chronic adrenal Addison ’ s disease — a disorder insuffi ciency in which the adrenal glands do not produce enough steroid hormones. 3 5. Recognize the signs and symptoms of Cushing ’ s syndrome and the result of too Adenoma — a benign much cortisol (noncancerous) tumor of glandular 6. Describe the pharmacologic and nonpharmacologic management of patients with origin. Cushing ’ s syndrome Adrenal insuffi ciency — a term 7. List management strategies for administration of glucocorticoid and mineralocorti- referring to a defi ciency in the levels of adrenal hormones. coid therapy to avoid development of adrenal disorders Aldosterone — the hormone produced by the adrenal glands that regulates the balance of sodium, he adrenal glands are an integral part of the endocrine system, secreting water, and potassium concentrations in the body. T hormones that act throughout the body to regulate functions and promote Corticotropin-releasing homeostasis. In addition to the neurotransmitters epinephrine and norepineph- hormone (CRH) — a hormone rine, the corticosteroids secreted by the adrenal glands are vital to a wide released by the hypothalamus that variety of physiological processes. -

GLI2 but Not GLI1/GLI3 Plays a Central Role in the Induction of Malignant Phenotype of Gallbladder Cancer
ONCOLOGY REPORTS 45: 997-1010, 2021 GLI2 but not GLI1/GLI3 plays a central role in the induction of malignant phenotype of gallbladder cancer SHU ICHIMIYA1, HIDEYA ONISHI1, SHINJIRO NAGAO1, SATOKO KOGA1, KUKIKO SAKIHAMA2, KAZUNORI NAKAYAMA1, AKIKO FUJIMURA3, YASUHIRO OYAMA4, AKIRA IMAIZUMI1, YOSHINAO ODA2 and MASAFUMI NAKAMURA4 Departments of 1Cancer Therapy and Research, 2Anatomical Pathology, 3Otorhinolaryngology and 4Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University, Fukuoka 812‑8582, Japan Received August 10, 2020; Accepted December 7, 2020 DOI: 10.3892/or.2021.7947 Abstract. We previously reported that Hedgehog (Hh) signal Introduction was enhanced in gallbladder cancer (GBC) and was involved in the induction of malignant phenotype of GBC. In recent Gallbladder cancer (GBC) is the seventh most common gastro- years, therapeutics that target Hh signaling have focused on intestinal carcinoma and accounts for 1.2% of all cancer cases molecules downstream of smoothened (SMO). The three tran- and 1.7% of all cancer-related deaths (1). GBC develops from scription factors in the Hh signal pathway, glioma-associated metaplasia to dysplasia to carcinoma in situ and then to invasive oncogene homolog 1 (GLI1), GLI2, and GLI3, function down- carcinoma over 5‑15 years (2). During this time, GBC exhibits stream of SMO, but their biological role in GBC remains few characteristic symptoms, and numerous cases have already unclear. In the present study, the biological significance of developed into locally advanced or metastasized cancer by the GLI1, GLI2, and GLI3 were analyzed with the aim of devel- time of diagnosis. Gemcitabine (GEM), cisplatin (CDDP), and oping novel treatments for GBC. -

Distinct Activities of Gli1 and Gli2 in the Absence of Ift88 and the Primary Cilia
Journal of Developmental Biology Article Distinct Activities of Gli1 and Gli2 in the Absence of Ift88 and the Primary Cilia Yuan Wang 1,2,†, Huiqing Zeng 1,† and Aimin Liu 1,* 1 Department of Biology, Eberly College of Sciences, Center for Cellular Dynamics, Huck Institute of Life Science, The Penn State University, University Park, PA 16802, USA; [email protected] (Y.W.); [email protected] (H.Z.) 2 Department of Occupational Health, School of Public Health, China Medical University, No.77 Puhe Road, Shenyang North New Area, Shenyang 110122, China * Correspondence: [email protected]; Tel.: +1-814-865-7043 † These authors contributed equally to this work. Received: 2 November 2018; Accepted: 16 February 2019; Published: 19 February 2019 Abstract: The primary cilia play essential roles in Hh-dependent Gli2 activation and Gli3 proteolytic processing in mammals. However, the roles of the cilia in Gli1 activation remain unresolved due to the loss of Gli1 transcription in cilia mutant embryos, and the inability to address this question by overexpression in cultured cells. Here, we address the roles of the cilia in Gli1 activation by expressing Gli1 from the Gli2 locus in mouse embryos. We find that the maximal activation of Gli1 depends on the cilia, but partial activation of Gli1 by Smo-mediated Hh signaling exists in the absence of the cilia. Combined with reduced Gli3 repressors, this partial activation of Gli1 leads to dorsal expansion of V3 interneuron and motor neuron domains in the absence of the cilia. Moreover, expressing Gli1 from the Gli2 locus in the presence of reduced Sufu has no recognizable impact on neural tube patterning, suggesting an imbalance between the dosages of Gli and Sufu does not explain the extra Gli1 activity. -

Hypothalamushypothalamus -- Pituitarypituitary -- Adrenaladrenal Glandsglands
HypothalamusHypothalamus -- pituitarypituitary -- adrenaladrenal glandsglands Magdalena Gibas-Dorna MD, PhD Dept. of Physiology University of Medical Sciences Poznań, Poland Hypothalamus - general director of the hormone system. At every moment, the hypothalamus analyses messages coming from: the brain and different regions of the body. Homeostatic functions of hypothalamus include maintaining a stable body temperature, controlling food intake, controlling blood pressure, ensuring a fluid balance, and even proper sleep patterns. Cell bodies of neurons that produce releasing/inhibiting hormones Hypothalamus HypothalamusHypothalamus releases Arterial flow Primary capillaries in median eminence hormones at Long Releasing Portal hormones Anterior veins median eminence pituitary hormone Releasing/ inhibiting hormones and sends to anterior pituitary ANTERIOR PITUITARY via portalportal veinvein. Secretory cells that produce anterior pituitary hormones Anterior pituitary hormones Venous outflow Gonadotropic Thyroid- Proactin hormones stimulating ACTH Growth (FSH and LH) hormone hormone ControlControl ofof pituitarypituitary hormonehormone secretionsecretion byby hypothalamushypothalamus • Secretion by the anterioranterior pituitarypituitary is controlled by hormones called hypothalamic releasing hormones and inhibitory hormones conducted to the anterior pituitary through hypothalamichypothalamic -- hypophysialhypophysial portalportal vesselsvessels .. • PosteriorPosterior pituitarypituitary secrets two hormones, which are synthesized within cell -

Mechanisms Acting on Hedgehog-Gli Pathway and Their Therapeutic Potential
From the Department of Biosciences and Nutrition Karolinska Institutet, Stockholm, Sweden MECHANISMS ACTING ON HEDGEHOG-GLI PATHWAY AND THEIR THERAPEUTIC POTENTIAL Ani Azatyan Stockholm 2020 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by Universitetsservice US-AB © Ani Azatyan, 2020 ISBN 978-91-7831-880-3 Mechanisms acting on Hedgehog-GLI pathway and their therapeutic potential THESIS FOR DOCTORAL DEGREE (Ph.D.) By Ani Azatyan Principal Supervisor: Opponent: Prof. Peter Zaphiropoulos Prof. Matthias Lauth Karolinska Institutet Philipps-University Marburg, Germany Department of Biosciences and Nutrition Center for Tumor Biology and Immunology Co-supervisor(s): Examination Board: Prof. Karl Ekwall Doc. Ning Xu Landén Karolinska Institutet Karolinska Institutet Department of Biosciences and Nutrition Department of Medicine, Division of Dermatology Doc. Vladimir Bykov Prof. Sonia Lain Karolinska Institutet Karolinska Institutet Department of Oncology-Pathology Department of Microbiology, Tumor and Cell Biology Prof. Ann-Kristin Östlund Farrants Stockholm University Department of Molecular Biosciences “I am among those who think that science has great beauty” - Marie Curie Abstract Hedgehog signaling is crucial for diverse aspects of animal development, essential in regulating many cellular processes and is largely implicated in various forms of human cancer. However, many aspects of Hedgehog signaling are not completely understood. This thesis aims to contribute towards a better understanding of the mechanisms acting on Hedgehog-GLI signaling and explore their possible therapeutic potential. PAPER I. We demonstrate that the small molecule RITA, a p53 activator, downregulates Hedgehog signaling in human medulloblastoma and rhabdomyosarcoma cells via JNK kinase and irrespective of p53. In vitro RITA enhanced the anti-proliferative effects of the GLI antagonist GANT61. -

The Role of Highly Selective Androgen Receptor (AR) Targeted
P h a s e I I S t u d y o f I t r a c o n a z o l e i n B i o c h e m i c a l R e l a p s e Version 4.0: October 8, 2014 CC# 125513 CC# 125513: Hedgehog Inhibition as a Non-Castrating Approach to Hormone Sensitive Prostate Cancer: A Phase II Study of Itraconazole in Biochemical Relapse Investigational Agent: Itraconazole IND: IND Exempt (IND 116597) Protocol Version: 4.0 Version Date: October 8, 2014 Principal Investigator: Rahul Aggarwal, M.D., HS Assistant Clinical Professor Division of Hematology/Oncology, Department of Medicine University of California San Francisco 1600 Divisadero St. San Francisco, CA94115 [email protected] UCSF Co-Investigators: Charles J. Ryan, M.D., Eric Small, M.D., Professor of Medicine Professor of Medicine and Urology Lawrence Fong, M.D., Terence Friedlander, M.D., Professor in Residence Assistant Clinical Professor Amy Lin, M.D., Associate Clinical Professor Won Kim, M.D., Assistant Clinical Professor Statistician: Li Zhang, Ph.D, Biostatistics Core RevisionHistory October 8, 2014 Version 4.0 November 18, 2013 Version 3.0 January 28, 2013 Version 2.0 July 16, 2012 Version 1.0 Phase II - Itraconazole Page 1 of 79 P h a s e I I S t u d y o f I t r a c o n a z o l e i n B i o c h e m i c a l R e l a p s e Version 4.0: October 8, 2014 CC# 125513 Protocol Signature Page Protocol No.: 122513 Version # and Date: 4.0 - October 8, 2014 1. -

Hedgehog Signalling in the Tumourigenesis and Metastasis of Osteosarcoma, and Its Potential Value in the Clinical Therapy Of
Yao et al. Cell Death and Disease (2018) 9:701 DOI 10.1038/s41419-018-0647-1 Cell Death & Disease REVIEW ARTICLE Open Access Hedgehog signalling in the tumourigenesis and metastasis of osteosarcoma, and its potential value in the clinical therapy of osteosarcoma Zhihong Yao1,LeiHan1, Yongbin Chen2,FeiHe3,BinSun3, Santosh kamar1, Ya Zhang1,YihaoYang1, Cao Wang1 and Zuozhang Yang1 Abstract The Hedgehog (Hh) signalling pathway is involved in cell differentiation, growth and tissue polarity. This pathway is also involved in the progression and invasion of various human cancers. Osteosarcoma, a subtype of bone cancer, is commonly seen in children and adolescents. Typically, pulmonary osteosarcoma metastases are especially difficult to control. In the present paper, we summarise recent studies on the regulation of osteosarcoma progression and metastasis by downregulating Hh signalling. We also summarise the crosstalk between the Hh pathway and other cancer-related pathways in the tumourigenesis of various cancers. We further summarise and highlight the therapeutic value of potential inhibitors of Hh signalling in the clinical therapy of human cancers. 1234567890():,; 1234567890():,; Facts Open questions ● The Hh pathway regulates the progression of ● How does the Hh pathway regulate the tumourigenic osteosarcoma. progression and invasion of human osteosarcoma? ● The Hh pathway is involved in the metastasis of ● How does the Hh pathway interact with other osteosarcoma into other organs, such as the lungs. cancer-related pathways in the progression and ● The Hh pathway crosstalks with other cancer-related metastasis of cancers? pathways in the tumourigenesis of cancers. ● Could the Hh pathway be used as a target or ● The therapeutic value of the Hh pathway in the biomarker in clinical therapy for human clinical therapy of osteosarcoma is summarised. -
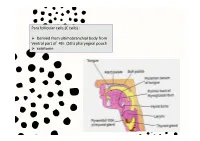
Para Follicular Cells (C Cells)
Para follicular cells (C cells) : Derived from ultimobranchial body from Ventral part of 4th (5th) pharyngeal pouch calcitonin Thyroglossal cyct: path of thyroid descending Position of occur: Inf. To the body of hyoid 50% Base of tongue Close to thyroid cartilage Thyroglossal fistula Abberant thyroid tissue: path of thyroid descending Base of tongue Histology of the thyroid gland Thyroid gland: parafollicular cells: Capsule / trabeculae Follicles / reticular fiber / Decreased Ca+ in blood by 2 ways: basal lamina Follicular cells/ basal lamina 1. Transport Ca from blood to musculoskeletal system parafollicular cells 2. Prevent bone absorption by osteoclast cells Colloid (hormone storage) Produce energy & temperature for body activity Histology of the thyroid gland Thyroid hormones: Tri-iodothyronin Tetra-iodothyronin Calcitonin Fenestrated capillary Synthesis and secretion of thyroid hormones T3 and T4 Synthesis and secretion of thyroid hormones T3 and T4 Synthesis and mechanism of action of calcitonin Gravies disease (exophthalmic goiter / toxic goiter): excessive amounts of thyroid hormones are released into the circulation detectable levels of autoantibodies abnormal immunoglobulins (IgG) bind to the TSH receptor in-creased thyroid hormone secretion Because of negative feedback, the levels of TSH in the circulation are usually normal Hypertrophy thyroid hormone Is abnormally high range increased metabolism Features: weight loss / excessive sweating / tachycardia /nervousness / protrusion of the eyeballs / retraction of the