Appendix, French Names, Supplement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
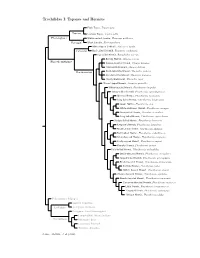
Topazes and Hermits
Trochilidae I: Topazes and Hermits Fiery Topaz, Topaza pyra Topazini Crimson Topaz, Topaza pella Florisuginae White-necked Jacobin, Florisuga mellivora Florisugini Black Jacobin, Florisuga fusca White-tipped Sicklebill, Eutoxeres aquila Eutoxerini Buff-tailed Sicklebill, Eutoxeres condamini Saw-billed Hermit, Ramphodon naevius Bronzy Hermit, Glaucis aeneus Phaethornithinae Rufous-breasted Hermit, Glaucis hirsutus ?Hook-billed Hermit, Glaucis dohrnii Threnetes ruckeri Phaethornithini Band-tailed Barbthroat, Pale-tailed Barbthroat, Threnetes leucurus ?Sooty Barbthroat, Threnetes niger ?Broad-tipped Hermit, Anopetia gounellei White-bearded Hermit, Phaethornis hispidus Tawny-bellied Hermit, Phaethornis syrmatophorus Mexican Hermit, Phaethornis mexicanus Long-billed Hermit, Phaethornis longirostris Green Hermit, Phaethornis guy White-whiskered Hermit, Phaethornis yaruqui Great-billed Hermit, Phaethornis malaris Long-tailed Hermit, Phaethornis superciliosus Straight-billed Hermit, Phaethornis bourcieri Koepcke’s Hermit, Phaethornis koepckeae Needle-billed Hermit, Phaethornis philippii Buff-bellied Hermit, Phaethornis subochraceus Scale-throated Hermit, Phaethornis eurynome Sooty-capped Hermit, Phaethornis augusti Planalto Hermit, Phaethornis pretrei Pale-bellied Hermit, Phaethornis anthophilus Stripe-throated Hermit, Phaethornis striigularis Gray-chinned Hermit, Phaethornis griseogularis Black-throated Hermit, Phaethornis atrimentalis Reddish Hermit, Phaethornis ruber ?White-browed Hermit, Phaethornis stuarti ?Dusky-throated Hermit, Phaethornis squalidus Streak-throated Hermit, Phaethornis rupurumii Cinnamon-throated Hermit, Phaethornis nattereri Little Hermit, Phaethornis longuemareus ?Tapajos Hermit, Phaethornis aethopygus ?Minute Hermit, Phaethornis idaliae Polytminae: Mangos Lesbiini: Coquettes Lesbiinae Coeligenini: Brilliants Patagonini: Giant Hummingbird Lampornithini: Mountain-Gems Tro chilinae Mellisugini: Bees Cynanthini: Emeralds Trochilini: Amazilias Source: McGuire et al. (2014).. -

Birds of Chile a Photo Guide
© Copyright, Princeton University Press. No part of this book may be 88 distributed, posted, or reproduced in any form by digital or mechanical 89 means without prior written permission of the publisher. WALKING WATERBIRDS unmistakable, elegant wader; no similar species in Chile SHOREBIRDS For ID purposes there are 3 basic types of shorebirds: 6 ‘unmistakable’ species (avocet, stilt, oystercatchers, sheathbill; pp. 89–91); 13 plovers (mainly visual feeders with stop- start feeding actions; pp. 92–98); and 22 sandpipers (mainly tactile feeders, probing and pick- ing as they walk along; pp. 99–109). Most favor open habitats, typically near water. Different species readily associate together, which can help with ID—compare size, shape, and behavior of an unfamiliar species with other species you know (see below); voice can also be useful. 2 1 5 3 3 3 4 4 7 6 6 Andean Avocet Recurvirostra andina 45–48cm N Andes. Fairly common s. to Atacama (3700–4600m); rarely wanders to coast. Shallow saline lakes, At first glance, these shorebirds might seem impossible to ID, but it helps when different species as- adjacent bogs. Feeds by wading, sweeping its bill side to side in shallow water. Calls: ringing, slightly sociate together. The unmistakable White-backed Stilt left of center (1) is one reference point, and nasal wiek wiek…, and wehk. Ages/sexes similar, but female bill more strongly recurved. the large brown sandpiper with a decurved bill at far left is a Hudsonian Whimbrel (2), another reference for size. Thus, the 4 stocky, short-billed, standing shorebirds = Black-bellied Plovers (3). -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

A 2010 Supplement to Ducks, Geese, and Swans of the World
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "The World’s Waterfowl in the 21st Century: A 2010 Supplement to Ducks, Geese, and Swans of the World" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 20. https://digitalcommons.unl.edu/biosciducksgeeseswans/20 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. The World’s Waterfowl in the 21st Century: A 200 Supplement to Ducks, Geese, and Swans of the World Paul A. Johnsgard Pages xvii–xxiii: recent taxonomic changes, I have revised sev- Introduction to the Family Anatidae eral of the range maps to conform with more current information. For these updates I have Since the 978 publication of my Ducks, Geese relied largely on Kear (2005). and Swans of the World hundreds if not thou- Other important waterfowl books published sands of publications on the Anatidae have since 978 and covering the entire waterfowl appeared, making a comprehensive literature family include an identification guide to the supplement and text updating impossible. -

Antarctic Primer
Antarctic Primer By Nigel Sitwell, Tom Ritchie & Gary Miller By Nigel Sitwell, Tom Ritchie & Gary Miller Designed by: Olivia Young, Aurora Expeditions October 2018 Cover image © I.Tortosa Morgan Suite 12, Level 2 35 Buckingham Street Surry Hills, Sydney NSW 2010, Australia To anyone who goes to the Antarctic, there is a tremendous appeal, an unparalleled combination of grandeur, beauty, vastness, loneliness, and malevolence —all of which sound terribly melodramatic — but which truly convey the actual feeling of Antarctica. Where else in the world are all of these descriptions really true? —Captain T.L.M. Sunter, ‘The Antarctic Century Newsletter ANTARCTIC PRIMER 2018 | 3 CONTENTS I. CONSERVING ANTARCTICA Guidance for Visitors to the Antarctic Antarctica’s Historic Heritage South Georgia Biosecurity II. THE PHYSICAL ENVIRONMENT Antarctica The Southern Ocean The Continent Climate Atmospheric Phenomena The Ozone Hole Climate Change Sea Ice The Antarctic Ice Cap Icebergs A Short Glossary of Ice Terms III. THE BIOLOGICAL ENVIRONMENT Life in Antarctica Adapting to the Cold The Kingdom of Krill IV. THE WILDLIFE Antarctic Squids Antarctic Fishes Antarctic Birds Antarctic Seals Antarctic Whales 4 AURORA EXPEDITIONS | Pioneering expedition travel to the heart of nature. CONTENTS V. EXPLORERS AND SCIENTISTS The Exploration of Antarctica The Antarctic Treaty VI. PLACES YOU MAY VISIT South Shetland Islands Antarctic Peninsula Weddell Sea South Orkney Islands South Georgia The Falkland Islands South Sandwich Islands The Historic Ross Sea Sector Commonwealth Bay VII. FURTHER READING VIII. WILDLIFE CHECKLISTS ANTARCTIC PRIMER 2018 | 5 Adélie penguins in the Antarctic Peninsula I. CONSERVING ANTARCTICA Antarctica is the largest wilderness area on earth, a place that must be preserved in its present, virtually pristine state. -

The Behavior and Ecology of Hermit Hummingbirds in the Kanaku Mountains, Guyana
THE BEHAVIOR AND ECOLOGY OF HERMIT HUMMINGBIRDS IN THE KANAKU MOUNTAINS, GUYANA. BARBARA K. SNOW OR nearly three months, 17 January to 5 April 1970, my husband and I F camped at the foot of the Kanaku Mountains in southern Guyana. Our camp was situated just inside the forest beside Karusu Creek, a tributary of Moco Moco Creek, at approximately 80 m above sea level. The period of our visit was the end of the main dry season which in this part of Guyana lasts approximately from September or October to April or May. Although we were both mainly occupied with other observations we hoped to accumulate as much information as possible on the hermit hummingbirds of the area, particularly their feeding niches, nesting and social organization. Previously, while living in Trinidad, we had studied various aspects of the behavior and biology of the three hermit hummingbirds resident there: the breeding season (D. W. Snow and B. K. Snow, 1964)) the behavior at singing assemblies of the Little Hermit (Phaethornis Zonguemareus) (D. W. Snow, 1968)) the feeding niches (B. K. Snow and D. W. Snow, 1972)) the social organization of the Hairy Hermit (Glaucis hirsuta) (B. K. Snow, 1973) and its breeding biology (D. W. Snow and B. K. Snow, 1973)) and the be- havior and breeding of the Guys’ Hermit (Phuethornis guy) (B. K. Snow, in press). A total of six hermit hummingbirds were seen in the Karusu Creek study area. Two species, Phuethornis uugusti and Phaethornis longuemureus, were extremely scarce. P. uugusti was seen feeding once, and what was presumably the same individual was trapped shortly afterwards. -

ON 1196 NEW.Fm
SHORT COMMUNICATIONS ORNITOLOGIA NEOTROPICAL 25: 237–243, 2014 © The Neotropical Ornithological Society NON-RANDOM ORIENTATION IN WOODPECKER CAVITY ENTRANCES IN A TROPICAL RAIN FOREST Daniel Rico1 & Luis Sandoval2,3 1The University of Nebraska-Lincoln, Lincoln, Nebraska. 2Department of Biological Sciences, University of Windsor, 401 Sunset Avenue, Windsor, ON, Canada, N9B3P4. 3Escuela de Biología, Universidad de Costa Rica, San Pedro, San José, Costa Rica, CP 2090. E-mail: [email protected] Orientación no al azar de las entradas de las cavidades de carpinteros en un bosque tropical. Key words: Pale-billed Woodpecker, Campephilus guatemalensis, Chestnut-colored Woodpecker, Celeus castaneus, Lineated Woodpecker, Dryocopus lineatus, Black-cheeked Woodpecker, Melanerpes pucherani, Costa Rica, Picidae. INTRODUCTION tics such as vegetation coverage of the nesting substrate, surrounding vegetation, and forest Nest site selection play’s one of the main roles age (Aitken et al. 2002, Adkins Giese & Cuth- in the breeding success of birds, because this bert 2003, Sandoval & Barrantes 2006). Nest selection influences the survival of eggs, orientation also plays an important role in the chicks, and adults by inducing variables such breeding success of woodpeckers, because the as the microclimatic conditions of the nest orientation positively influences the microcli- and probability of being detected by preda- mate conditions inside the nest cavity (Hooge tors (Viñuela & Sunyer 1992). Although et al. 1999, Wiebe 2001), by reducing the woodpecker nest site selections are well estab- exposure to direct wind currents, rainfalls, lished, the majority of this information is and/or extreme temperatures (Ardia et al. based on temperate forest species and com- 2006). Cavity entrance orientation showed munities (Newton 1998, Cornelius et al. -

Brazil's Eastern Amazonia
The loud and impressive White Bellbird, one of the many highlights on the Brazil’s Eastern Amazonia 2017 tour (Eduardo Patrial) BRAZIL’S EASTERN AMAZONIA 8/16 – 26 AUGUST 2017 LEADER: EDUARDO PATRIAL This second edition of Brazil’s Eastern Amazonia was absolutely a phenomenal trip with over five hundred species recorded (514). Some adjustments happily facilitated the logistics (internal flights) a bit and we also could explore some areas around Belem this time, providing some extra good birds to our list. Our time at Amazonia National Park was good and we managed to get most of the important targets, despite the quite low bird activity noticed along the trails when we were there. Carajas National Forest on the other hand was very busy and produced an overwhelming cast of fine birds (and a Giant Armadillo!). Caxias in the end came again as good as it gets, and this time with the novelty of visiting a new site, Campo Maior, a place that reminds the lowlands from Pantanal. On this amazing tour we had the chance to enjoy the special avifauna from two important interfluvium in the Brazilian Amazon, the Madeira – Tapajos and Xingu – Tocantins; and also the specialties from a poorly covered corner in the Northeast region at Maranhão and Piauí states. Check out below the highlights from this successful adventure: Horned Screamer, Masked Duck, Chestnut- headed and Buff-browed Chachalacas, White-crested Guan, Bare-faced Curassow, King Vulture, Black-and- white and Ornate Hawk-Eagles, White and White-browed Hawks, Rufous-sided and Russet-crowned Crakes, Dark-winged Trumpeter (ssp. -

Redalyc.The Role of Historical and Local Factors in Determining Species
Revista de Biología Tropical ISSN: 0034-7744 [email protected] Universidad de Costa Rica Costa Rica Barrantes, Gilbert The role of historical and local factors in determining species composition of the highland avifauna of Costa Rica and Western Panamá Revista de Biología Tropical, vol. 57, núm. 1, noviembre, 2009, pp. 333-349 Universidad de Costa Rica San Pedro de Montes de Oca, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44918950029 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative The role of historical and local factors in determining species composition of the highland avifauna of Costa Rica and Western Panamá Gilbert Barrantes Escuela de biología, Universidad de Costa Rica, 11501-2060, San José, Costa Rica; [email protected] Received 30-VI-2007. Corrected 09-X-2008. Accepted 18-XI-2008. Abstract: The formation of the mountain ranges of Costa Rica and western Panamá, as well as the cold climatic conditions that prevailed during the upper Pleistocene, played a crucial role in determining the bird species composition of the highlands in this region. Glacial conditions favored dispersal movements of bird species from the Andes, and from the Neartic region. Subsequent inter-glacial conditions reduced the connectivity between neotropical highlands (e.g., Talamanca-Andes), and between neotropical highlands and Neartic temper- ate region, isolating recently established populations from the ancestral populations, and promoting speciation. -

Diet of Breeding White-Throated and Black Swifts in Southern California
DIET OF BREEDING WHITE-THROATED AND BLACK SWIFTS IN SOUTHERN CALIFORNIA ALLISON D. RUDALEVIGE, DESSlE L. A. UNDERWOOD, and CHARLES T. COLLINS, Department of BiologicalSciences, California State University,Long Beach, California 90840 (current addressof Rudalevige:Biology Department, Universityof California,Riverside, California 92521) ABSTRACT: We analyzed the diet of nestling White-throated(Aeronautes saxatalis) and Black Swifts (Cypseloidesniger) in southern California. White- throatedSwifts fed their nestlingson bolusesof insectsmore taxonomicallydiverse, on average(over 50 arthropodfamilies represented), than did BlackSwifts (seven arthropodfamilies, primarfiy ants). In some casesWhite-throated Swift boluses containedprimarily one species,while other bolusesshowed more variation.In contrast,all BlackSwift samplescontained high numbersof wingedants with few individualsof other taxa. Our resultsprovide new informationon the White-throated Swift'sdiet and supportprevious studies of the BlackSwift. Swiftsare amongthe mostaerial of birds,spending most of the day on the wing in searchof their arthropodprey. Food itemsinclude a wide array of insectsand some ballooningspiders, all gatheredaloft in the air column (Lack and Owen 1955). The food habitsof a numberof speciesof swifts have been recorded(Collins 1968, Hespenheide1975, Lack and Owen 1955, Marfn 1999, Tarburton 1986, 1993), but there is stilllittle informa- tion availablefor others, even for some speciesthat are widespreadand common.Here we providedata on the prey sizeand compositionof food broughtto nestlingsof the White-throated(Aerona u tes saxa talis) and Black (Cypseloidesniger) Swifts in southernCalifornia. The White-throatedSwift is a commonresident that nestswidely in southernCalifornia, while the Black Swift is a local summerresident, migrating south in late August (Garrettand Dunn 1981, Foersterand Collins 1990). METHODS When feedingyoung, swifts of the subfamiliesApodinae and Chaeturinae return to the nest with a bolusof food in their mouths(Collins 1998). -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Diversity and Clade Ages of West Indian Hummingbirds and the Largest Plant Clades Dependent on Them: a 5–9 Myr Young Mutualistic System
bs_bs_banner Biological Journal of the Linnean Society, 2015, 114, 848–859. With 3 figures Diversity and clade ages of West Indian hummingbirds and the largest plant clades dependent on them: a 5–9 Myr young mutualistic system STEFAN ABRAHAMCZYK1,2*, DANIEL SOUTO-VILARÓS1, JIMMY A. MCGUIRE3 and SUSANNE S. RENNER1 1Department of Biology, Institute for Systematic Botany and Mycology, University of Munich (LMU), Menzinger Str. 67, 80638 Munich, Germany 2Department of Biology, Nees Institute of Plant Biodiversity, University of Bonn, Meckenheimer Allee 170, 53113 Bonn, Germany 3Museum of Vertebrate Zoology and Department of Integrative Biology, University of California, 3101 Valley Life Sciences Building, Berkeley, CA 94720-3160, USA Received 23 September 2014; revised 10 November 2014; accepted for publication 12 November 2014 We analysed the geographical origins and divergence times of the West Indian hummingbirds, using a large clock-dated phylogeny that included 14 of the 15 West Indian species and statistical biogeographical reconstruction. We also compiled a list of 101 West Indian plant species with hummingbird-adapted flowers (90 of them endemic) and dated the most species-rich genera or tribes, with together 41 hummingbird-dependent species, namely Cestrum (seven spp.), Charianthus (six spp.), Gesnerieae (75 species, c. 14 of them hummingbird-pollinated), Passiflora (ten species, one return to bat-pollination) and Poitea (five spp.), to relate their ages to those of the bird species. Results imply that hummingbirds colonized the West Indies at least five times, from 6.6 Mya onwards, coming from South and Central America, and that there are five pairs of sister species that originated within the region.