Reactions and Characterization of Compounds Containing Tungsten Halide Cluster Species Ronald Dean Hogue Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Data Sheet
SAFETY DATA SHEET Revision Date 14-Feb-2020 Revision Number 2 1. Identification Product Name Silver bromide Cat No. : 12110 CAS-No 7785-23-1 Synonyms None Recommended Use Laboratory chemicals. Uses advised against Food, drug, pesticide or biocidal product use. Details of the supplier of the safety data sheet Company Alfa Aesar Thermo Fisher Scientific Chemicals, Inc. 30 Bond Street Ward Hill, MA 01835-8099 Tel: 800-343-0660 Fax: 800-322-4757 Email: [email protected] www.alfa.com Emergency Telephone Number During normal business hours (Monday-Friday, 8am-7pm EST), call (800) 343-0660. After normal business hours, call Carechem 24 at (866) 928-0789. 2. Hazard(s) identification Classification Classification under 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Based on available data, the classification criteria are not met Label Elements None required Hazards not otherwise classified (HNOC) None identified 3. Composition/Information on Ingredients Component CAS-No Weight % Silver bromide (AgBr) 7785-23-1 99 ______________________________________________________________________________________________ Page 1 / 6 Silver bromide Revision Date 14-Feb-2020 ______________________________________________________________________________________________ 4. First-aid measures Eye Contact Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. Get medical attention. Skin Contact Wash off immediately with soap and plenty of water while removing all contaminated clothes and shoes. Get medical attention. Inhalation Remove from exposure, lie down. Remove to fresh air. Get medical attention. Ingestion Clean mouth with water. Get medical attention. Most important symptoms and No information available. effects Notes to Physician Treat symptomatically 5. Fire-fighting measures Suitable Extinguishing Media Water spray. -

Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane
molecules Article Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane Andrew J. Peloquin 1, Srikar Alapati 2, Colin D. McMillen 1, Timothy W. Hanks 2 and William T. Pennington 1,* 1 Department of Chemistry, Clemson University, Clemson, SC 29634, USA; [email protected] (A.J.P.); [email protected] (C.D.M.) 2 Department of Chemistry, Furman University, Greenville, SC 29613, USA; [email protected] (S.A.); [email protected] (T.W.H.) * Correspondence: [email protected] Abstract: Through variations in reaction solvent and stoichiometry, a series of S-diiodine adducts of 1,3- and 1,4-dithiane were isolated by direct reaction of the dithianes with molecular diiodine in solution. In the case of 1,3-dithiane, variations in reaction solvent yielded both the equatorial and the axial isomers of S-diiodo-1,3-dithiane, and their solution thermodynamics were further studied via DFT. Additionally, S,S’-bis(diiodo)-1,3-dithiane was also isolated. The 1:1 cocrystal, (1,4-dithiane)·(I2) was further isolated, as well as a new polymorph of S,S’-bis(diiodo)-1,4-dithiane. Each structure showed significant S···I halogen and chalcogen bonding interactions. Further, the product of the diiodine-promoted oxidative addition of acetone to 1,4-dithiane, as well as two new cocrystals of 1,4-dithiane-1,4-dioxide involving hydronium, bromide, and tribromide ions, was isolated. Keywords: crystal engineering; chalcogen bonding; halogen bonding; polymorphism; X-ray diffraction Citation: Peloquin, A.J.; Alapati, S.; McMillen, C.D.; Hanks, T.W.; Pennington, W.T. -

PAPERS READ BEFORE the CHEMICAL SOCIETY. XXII1.-On
View Article Online / Journal Homepage / Table of Contents for this issue 773 PAPERS READ BEFORE THE CHEMICAL SOCIETY. XXII1.-On Tetrabromide of Carbon. No. II. By THOMASBOLAS and CHARLESE. GROVES. IN a former paper* we described several methods for the preparation of the hitherto unknown tetrabromide of carbon, and in the present communication we desire to lay before the Society the results of our more recent experiments. In addition to those methods of obtaining the carbon tetrabromide, which we have already published, the fol- lowing are of interest, either from a theoretical point of view, or as affording advantageous means for the preparation of that substance. Action of Bromine on Carbon Disulphide. Our former statement that? bromine had no action on carbon disul- phide requires some modification, as we find that when it is heated to 180" or 200" for several hundred hours with bromine free from both chlorine and iodine, and the contents of the tubes are neutralised and distilled in the usual way, a liquid is obtained, which consists almost entirely of unaltered carbon disulphide ; but when this is allowed to evaporate spontaneously, a small quantity of a crystalline substance is left, which has the appearance and properties of carbon tetrabromide. The length of time required for this reaction, and the very small relative amount of substance obtained, would, however, render this Published on 01 January 1871. Downloaded by Brown University 25/10/2014 10:39:25. quite inapplicable as a process for the preparation of the tetra- bromide. Action of Bromine on Carbon Disdphide in, presence of Certain Bromides. -

Safety Data Sheet
SAFETY DATA SHEET According to JIS Z 7253:2019 Revision Date 21-Dec-2020 Version 4.02 Section 1: PRODUCT AND COMPANY IDENTIFICATION Product name Silver(I) Bromide Product code 198-00732,190-00731 Manufacturer FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-5964 Supplier FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome, Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-2029 Emergency telephone number +81-6-6203-3741 / +81-3-3270-8571 Recommended uses and For research use only restrictions on use Section 2: HAZARDS IDENTIFICATION GHS classification Classification of the substance or mixture Short-term (acute) hazardous to the aquatic environment Category 1 Long-term (chronic) hazardous to the aquatic environment Category 1 Pictograms Signal word Warning Hazard statements H400 - Very toxic to aquatic life H410 - Very toxic to aquatic life with long lasting effects Precautionary statements-(Prevention) • Avoid release to the environment Precautionary statements-(Response) • Collect spillage Precautionary statements-(Storage) • Not applicable Precautionary statements-(Disposal) • Dispose of contents/container to an approved waste disposal plant Others Other hazards Not available Section 3: COMPOSITION/INFORMATION ON INGREDIENTS __________________________________________________________________________________________ Page 1 / 6 W01W0119-0073 JGHEEN Silver(I) Bromide Revision Date 21-Dec-2020 __________________________________________________________________________________________ Single Substance or Mixture Substance Formula AgBr Chemical Name Weight-% Molecular weight ENCS ISHL No. CAS RN Silver bromide 97.0 187.77 (1)-2 公表 7785-23-1 Impurities and/or Additives: Not applicable Section 4: FIRST AID MEASURES Inhalation Remove to fresh air. -

Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

Pp-03-25-New Dots.Qxd 10/23/02 2:16 PM Page 136
pp-03-25-new dots.qxd 10/23/02 2:16 PM Page 136 136 BROMIC ACID / BROMINE BROMIC ACID [7789–31–3] Formula: HBrO3; MW 128.91 Uses Bromic acid is used as an oxidizing agent; and also as intermediate in the preparation of dyes and pharmaceuticals . Physical Properties Unstable compound; stable only in dilute aqueous solutions; solution turns yellow on standing; decomposes when heated to 100°C. Preparation Bromic acid is prepared by adding sulfuric acid to barium bromate. Ba(BrO3)2 + H2SO4 → 2HBrO3 + BaSO4 The product is distilled and absorbed in water. A 50% solution may be obtained by slow evaporation of the dilute solution in vacuum at –12°C. Toxicity Contact with skin and eyes can cause severe irritation. BROMINE [7726–95–6] Symbol Br; atomic number 35; atomic weight 79.904; a halogen group ele- ment; electron affinity 3.36359 eV; electronegativity 2.8; electron configura- tion [Ar] 3d104s24p5; most stable valence states –1 and +5, less stable valence states +1 and +3; a diatomic molecule (Br2) in liquid and vapor states over a wide range of temperature; two stable isotopes, Br–79 (50.57%) and Br–81 (49.43%). Occurrence and Uses Bromine occurs in nature as bromide in many natural brine wells and salt deposits. It also is found in seawater at a concentration of 85 mg/L. The ele- ment was discovered by A. J. Balard and C. Lowig, independently in 1826. Bromine is used in bleaching fibers and as a disinfectant for water purifica- tion. Other applications are in organic synthesis as an oxidizing or brominat- ing agent; in the manufacture of ethylene dibromide, methyl bromide and other bromo compounds for dyes and pharmaceutical uses; as a fire retardant for plastics; and in chemical analysis. -

Attached Are the MSDS Lists for Various Chemicals Used During the Wet Plate Collodion / Platinum Palladium Courses at Oxbow
Attached are the MSDS lists for various chemicals used during the Wet Plate Collodion / Platinum Palladium courses at OxBow. Many of the materials for this course are highly toxic and therefore thorough communication and understanding about this aspect of wet plate collodion. Safety is a necessity. It is our first priority and concern to having a productive community throughout our courses. Please contact us should you have any questions or concerns, we will be happy to answer. Thank you, Jaclyn Silverman and Robert Clarke-Davis MSDS Lists: Wet-plate collodion * you will be required to use nitrile gloves, there is no exception to this rule. SAFETY DATA SHEET Preparation Date: No data available Revision Date: 6/17/2015 Revision Number: G1 Product identifier Product code: CO120 Product Name: COLLODION, USP Other means of identification Synonyms: No information available CAS #: Mixture RTECS # Not available CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: No information available. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute toxicity - Oral Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2 Reproductive toxicity Category 1B Specific target organ toxicity (single exposure) Category 3 Specific target organ toxicity (repeated exposure) Category 1 Flammable liquids Category 1 Label elements Product code: CO120 Product name: COLLODION, USP 1 / 16 Danger Hazard statements Harmful if swallowed Causes skin irritation Causes serious eye irritation May damage fertility or the unborn child May cause respiratory irritation. -

Iodine Monobromide Original Commentary
IODINE MONOBROMIDE 1 Iodine Monobromide O OH t 1. IBr, PhMe O -BuO O –80 to –85 °C (2) BrI 2. K2CO3, MeOH 71% OBn 13.9:1 [7789-33-5] Brl (MW 206.80) OBn InChI = 1S/BrI/c1-2 InChIKey = CBEQRNSPHCCXSH-UHFFFAOYAO (diastereoselective cyclizations of homoallylic carbonates; elec- trophilic addition to alkenes; -bromination of steroidal aldehy- Table 1 Regiochemistry of IBr addition to alkenes des and ketones;, cleavage of carbon–metal bonds) Alkene % Markovnikov % anti-Markovnikov Physical Data: mp 40 ◦ C; bp 116 ◦C (dec); d 4.416 g cm−3. Solubility: sol alcohol, ether, CS2, acetic acid, acetone, CH2Cl2, MeCH=CH2 65 35 MeCN, DME, toluene (but with limited solubility below –80 ErCH=CH2 45 55 ◦ C); soluble in H2O, but hydrolyzes to HBr and IOH. i-PrCH=CH2 15 85 Form Supplied in: brownish-black crystals or very hard, black t-BuCH=CH2 0 100 solid; widely available (usually 97–98% purity). PhCH=CH2 100 0 Handling, Storage, and Precautions: corrosive solid and vapor; readily absorbed through skin and mucous membranes; use in a fume hood and wear gloves and appropriate protective clothing; air-, moisture-, and light-sensitive; should be stored refrigerated (under N ) in a tightly sealed amber bottle. Forms explosive 2 mixtures with potassium and sodium, and reacts extremely -Bromination of Steroidal Aldehydes and Ketones. Io- exothermically with phosphorus and tin. dine monobromide is an effective monobrominating reagent for steroidal aldehydes (eq 3). The ratio of diastereomeric products is in part a function of the reaction site’s proximity to an asymmet- Original Commentary ric center, with -orientation favored for the entering bromine. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product identifier Silver Bromide Other means of identification SDS number 2AL Materion Code 2AL CAS number 7785-23-1 Manufacturer/Importer/Supplier/Distributor information Manufacturer Company name Materion Advanced Chemicals Inc. Address 407 N 13th Street 1316 W. St. Paul Avenue Milwaukee, WI 53233 United States Telephone 414.212.0257 E-mail [email protected] Contact person Noreen Atkinson Emergency phone number Chemtrec 800.424.9300 2. Hazard(s) identification Physical hazards Not classified. Health hazards Not classified. Environmental hazards Hazardous to the aquatic environment, acute Category 1 hazard Hazardous to the aquatic environment, Category 1 long-term hazard OSHA defined hazards Not classified. Label elements Signal word Warning Hazard statement Very toxic to aquatic life. Very toxic to aquatic life with long lasting effects. Precautionary statement Prevention Avoid release to the environment. Response Collect spillage. Storage Store away from incompatible materials. Disposal Dispose of contents/container in accordance with local/regional/national/international regulations. Hazard(s) not otherwise None known. classified (HNOC) Supplemental information None. 3. Composition/information on ingredients Substances Material name: Silver Bromide SDS US 2AL Version #: 02 Revision date: 01-15-2018 Issue date: 06-05-2015 1 / 6 Chemical name Common name and synonyms CAS number % Silver Bromide 7785-23-1 90 - 100 *Designates that a specific chemical identity and/or percentage of composition has been withheld as a trade secret. 4. First-aid measures Inhalation Move to fresh air. Call a physician if symptoms develop or persist. Skin contact Wash off with soap and water. Get medical attention if irritation develops and persists. -

Hazardous Substances (Chemicals) Transfer Notice 2006
16551655 OF THURSDAY, 22 JUNE 2006 WELLINGTON: WEDNESDAY, 28 JUNE 2006 — ISSUE NO. 72 ENVIRONMENTAL RISK MANAGEMENT AUTHORITY HAZARDOUS SUBSTANCES (CHEMICALS) TRANSFER NOTICE 2006 PURSUANT TO THE HAZARDOUS SUBSTANCES AND NEW ORGANISMS ACT 1996 1656 NEW ZEALAND GAZETTE, No. 72 28 JUNE 2006 Hazardous Substances and New Organisms Act 1996 Hazardous Substances (Chemicals) Transfer Notice 2006 Pursuant to section 160A of the Hazardous Substances and New Organisms Act 1996 (in this notice referred to as the Act), the Environmental Risk Management Authority gives the following notice. Contents 1 Title 2 Commencement 3 Interpretation 4 Deemed assessment and approval 5 Deemed hazard classification 6 Application of controls and changes to controls 7 Other obligations and restrictions 8 Exposure limits Schedule 1 List of substances to be transferred Schedule 2 Changes to controls Schedule 3 New controls Schedule 4 Transitional controls ______________________________ 1 Title This notice is the Hazardous Substances (Chemicals) Transfer Notice 2006. 2 Commencement This notice comes into force on 1 July 2006. 3 Interpretation In this notice, unless the context otherwise requires,— (a) words and phrases have the meanings given to them in the Act and in regulations made under the Act; and (b) the following words and phrases have the following meanings: 28 JUNE 2006 NEW ZEALAND GAZETTE, No. 72 1657 manufacture has the meaning given to it in the Act, and for the avoidance of doubt includes formulation of other hazardous substances pesticide includes but -
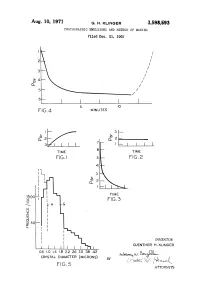
Aug. 10, 1971 G. H. KLINGER 3,598,593 PHOTOGRAPHIC EMUISIONS and METHOD of MAKING Filed Dec
Aug. 10, 1971 G. H. KLINGER 3,598,593 PHOTOGRAPHIC EMUISIONS AND METHOD OF MAKING Filed Dec. 21, 1965 FG4 MINUTES TIME FG.2 8 OO INVENTOR GUENTHER H. KLINGER O 6 .O 1.4 .8 222.2 262.6 3.O 388 4.42 w). (e 38. CRYSTAL DIAMETER (MICRONs) by f 1 ( i I F.G. 5 v. T. ATTORNEYs 3,598,593 United States Patent Office Patented Aug. 10, 1971 1. 2 In the general case of ammonia emulsions, the am 3,598,593 monia can either be added with the silver nitrate solu PHOTOGRAPHC EMULSIONS AND METHOD OF MAKNG tion or it may be added after mixing the silver nitrate and Guenther Harald Kinger, Binghamton, N.Y., assignor to part or all of the halide solution. GAF Corporation, New York, N.Y. 5 According to further aspects of the present invention, Filed Dec. 21, 1965, Ser. No. 515,391 after it was found that it is not essential to maintain a Int, C. G03c 1/02 substantial excess of halide ions, a more detailed in U.S. C. 96-94 8 Claims vestigation on correlation of crystal growth and ion con centrations in the photographic emulsions was under 10 taken. As a result of this investigation it was further found ABSTRACT OF THE DISCLOSURE that it is possible to use only a very small excess of Preparation of gelatin-silver bromide photographic halide ions and still obtain excellent products. It was also emulsions, involving precipitation of the silver bromide by found that even a small excess of silver ions could be admixture of aqueous solutions of a water soluble silver employed during the crystal silver halide grain formation.