The Oxidation of Alpha and Beta Glucose and a Study of the Isomeric Forms of the Sugar in Solution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduetion
THE CONCENTFL4TION OF RADIUM AND MESO- THORIUM BY FRACTIONAL CRYSTALLIZATION* BY JOHN I,. NIERMAN Introduetion MarkwaldlO and Soddyl' have shown independently that mesothorium is absolutely identical in chemical nature with radium and cannot be separated therefrom.** In consequence all radium separated from uranium minerals containing thorium, contains also the mesothorium in the mineral, and all preparations of mesothorium contain the radium that is present in the mineral from which the thorium is derived. In the extraction and recovery of the minute quantities of mesothorium and radium present in radioactive minerals, these elements become associated with barium and follow the barium throughout the process. The refining of mesothorium and radium then consists in separating these elements from barium, the method generally followed being fractional crystallization of the barium solution, first as chloride, and later as bromide. The mesothorium and radium continue to be enriched in the crystal fractions, and reduced in the succes- sive mother liquors. t In practice, l2 a fair concentration of acid is maintained throughout the chloride and bromide systems, for the reason ' that the factor of enrichment of mesothorium radium chloride from barium chloride and also of mesothorium radium bromide from barium bromide is regarded as more favorable in acid than in neutral solutions. While it has been shown3 that the crystallization factor is higher for bromides than for chlorides, the effect of the acidity of the solutions on the progress of * Abstract of a thesis submitted in partial fulfilment of the requirements for the degree of Master of Arts in the Graduate, School of the University of Missouri, August, 1919. -

Effects of Intravenous Injections of Radium Bromide. by R
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central EFFECTS OF INTRAVENOUS INJECTIONS OF RADIUM BROMIDE. BY R. BURTON-OPITZ AND GUSTAVE M. MEYER. (From the Laboratories of Physiology and Physiological Chemistry of Colum- bia University, at the College of Physicians and Surgeons, New York.) PLATE XVI. The present study was undertaken with a view of determining in a general way the effects of intravenous administration of ra- dium upon the circulation and respiration. The problem was sug- gested to us by Dr. William J. Gies, under whose guidance a number of researches, dealing with the more extensive question of the action of radium upon animal and vegetable cells, have re- cently been carried on in the laboratories of Columbia University.1 For the radium used in these experiments we are greatly in- debted to Mr. Hugo Lieber. It was supplied to us in the form of the bromide, in preparations of 240 , iooo, and io,ooo activities. The strength of the solution used was the same in all cases. It contained 45 rag. of the dry substance in 25 c. c. of the solvent; each cubic centimeter of the solution, therefore, contained 1.8 rag. of the radium preparation. The amount of the radium present varies directly with the ra- dio-activity. Preparations of ~,5oo,ooo activity are said to repre- sent pure radium bromide3 Taking this figure as the standard of purity, ~ .8 rag. of the radium preparation of io,ooo activity contained approximately only o.o~ 26 mg., the same quantity of the preparation of ~ooo activity contained o.ooi26 rag. -

United States Patent (19) 11) 4,078,045 Nakayama Et Al
United States Patent (19) 11) 4,078,045 Nakayama et al. 45) Mar. 7, 1978 (54) PROCESS FOR PRODUCING CARBONYL (56) References Cited SULFIDE U.S. PATENT DOCUMENTS (75) Inventors: Yoshiki Nakayama, Shimizu; Hironobu Sano, Fuji; Sataro 2,992,897 7/1961 Applegathet al................... 423/416 Okamura; Kazunari Hirao, both of 3,764,661 10/1973 Kanazawa et al. .................. 423/416 Shizuoka, all of Japan Primary Examiner-Earl C. Thomas (73) Assignee: Ihara Chemical Industry Co., Ltd., Attorney, Agent, or Firm-Oblon, Fisher, Spivak, Tokyo, Japan McClelland & Maier 21) Appl. No.: 736,334 57 ABSTRACT 22) Filed: Oct. 28, 1976 Carbonyl sulfide is produced by reacting carbon mon (30) Foreign Application Priority Data oxide with sulfur in the presence of an alkaline earth metal compound selected from the group consisting of Apr. 28, 1976 Japan .................................. 51-48944 calcium, strontium or barium sulfides sulfates and ha (51) Int. C.’.............................................. C01B31/26 lides. 52) U.S. Cl. .................................................... 423/416 (58) Field of Search ................................. 423/414-416 6 Claims, 1 Drawing Figure U.S. Patent March 7, 1978 4,078,045 -a COS 4,078,045 1 2 However, when the conversion is low, carbon mon PROCESS FOR PRODUCING CARBONYL oxide of the unreacted material contained in the reac SULFIDE tion mixture gas at high ratio should be separated and recovered from carbonyl sulfide. Accordingly, the pro BACKGROUND OF THE INVENTION cess is not satisfactory for the industrial operation. The present invention relates to an improved process On the other hand, in the process for reacting carbon for producing carbonyl sulfide which is useful as the monoxide with sulfur in the presence of the alkali metal intermediate for agriculture chemicals, medicines and sulfide such as sodium or potassium sulfide, the catalytic other chemical compounds. -

Download Author Version (PDF)
Analytical Methods Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/methods Page 1 of 21 Analytical Methods 1 2 3 4 Multi-element analysis of sulfuric acid by oaTOF-ICP-MS after matrix 5 6 modification with barium bromide 7 8 9 10 11 Lenka Husáková*, Iva Urbanová, Tereza Šídová, Tomáš Mikysek 12 13 14 Department of Analytical Chemistry, Faculty of Chemical Technology, University of Pardubice, 15 16 Pardubice, Studentska 573 HB/D, CZ-532 10, Czech Republic 17 18 19 20 21 * Corresponding author: Tel. +420 466 037 029 fax: +420 466 037 068 Manuscript 22 23 E-mail address: [email protected] 24 25 26 27 28 29 30 31 Accepted 32 33 34 35 36 37 38 39 40 Methods 41 42 43 44 45 46 47 48 49 50 51 Analytical 52 53 54 55 56 57 58 59 60 1 Analytical Methods Page 2 of 21 1 2 3 Abstract 4 5 6 In this work a novel method for simultaneous multi-element analysis of sulfuric acid by 7 8 inductively coupled plasma orthogonal acceleration time-of-flight mass spectrometry (oaTOF- 9 10 ICP-MS) after matrix modification with barium bromide was introduced. -

Chemical Formula of Binary Ionic Compounds – Sheet 1 the Combining Power Or Valency of Silver Is Always 1
Chemical Formula of Binary Ionic Compounds – Sheet 1 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. Binary compound Formula 1 potassium fluoride 26 calcium sulfide 2 calcium chloride 27 lithium bromide 3 barium bromide 28 nickel sulfide 4 silver sulfide 29 zinc phosphide 5 aluminium iodide 30 barium iodide 6 potassium iodide 31 caesium chloride 7 lead(IV) oxide 32 copper bromide 8 zinc nitride 33 sodium nitride 9 silver iodide 34 silver chloride 10 barium fluoride 35 sodium hydride 11 lead(II) iodide 36 potassium nitride 12 silver fluoride 37 cobalt chloride 13 sodium sulfide 38 magnesium sulfide 14 sodium bromide 39 potassium chloride 15 calcium oxide 40 calcium bromide 16 zinc fluoride 41 iron(III) oxide 17 strontium phosphide 42 aluminium fluoride 18 barium sulfide 43 magnesium bromide 19 aluminium oxide 44 iron(III) chloride 20 aluminium chloride 45 barium nitride 21 aluminium sulfide 46 sodium fluoride 22 lead(II) oxide 47 lithium fluoride 23 barium chloride 48 lithium iodide 24 copper chloride 49 lithium hydride 25 barium phosphide 50 potassium oxide “Aluminum” and “cesium” are commonly used alternative spellings for "aluminium" and "caesium that are used in the US. May be freely copied for educational use. ©www.chemicalformula.org Chemical Formula of Binary Ionic Compounds – Sheet 2 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. -

Effect of the Temperature and Solvents on the Solvolysis of Barium Bromide in Aqueous-Organic Solutions: Volumetric and Viscometric Study
Article Volume 12, Issue 1, 2022, 339 - 350 https://doi.org/10.33263/BRIAC121.339350 Effect of the Temperature and Solvents on the Solvolysis of Barium Bromide in Aqueous-Organic Solutions: Volumetric and Viscometric Study Sudhansu Sekhar Pattnaik 1 , Binita Nanda 2 , Biswajit Dalai 1 , Braja B. Nanda 3, * 1 Department of Physics, GIET University, Gunupur, Odisha, India; [email protected] (S.S.P.); 2 Department of Chemistry, ITER, Sikshya ‘O’ Anusandhan (Deemed to be University), Bhubaneswar-751030, India; [email protected] (B.N.); 3 P.G. Department of Chemistry, Vikram Deb Autonomous College, Jeypore-764001, Odisha, India; [email protected] (B.B.N.); * Correspondence: [email protected]; Scopus Author ID 8431581400 Received: 23.02.2021; Revised: 2.04.2021; Accepted: 7.04.2021; Published: 20.04.2021 Abstract: Volumetric and viscometric properties of solutions containing barium bromide in an aqueous solution of ethylene glycol and 1,4-dioxane have been discussed at different temperatures such as 298.15K 303.15K, 308.15K, and 313.15K. The Masson’s equation was used to determine the apparent 0 0 molar volume, 푉휙, standard partial molar volume, 푉휙 , molar expansibilities,퐸휙 by taking the density data. The values of viscosity and density were used in the Jones-Dole equation to find the viscosity B coefficients, which were used to estimate the ion-solvent interactions. The values of Hepler’s constant 2 0 2 (휕 푉휙 / 휕T )p and the viscosity B-coefficients have been used to deduce the solvent structure-promoting or structure breaking tendency of the salt in the studied mixtures. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

The Radiochemistry of Radium Commllleeon NUCLEAR SCIENCE
National .—. Academy d Sciences NationalI Research Council m NUCLEAR SCIENCE SERIES The Radiochemistry of Radium COMMlllEEON NUCLEAR SCIENCE B.K- Aufmn,Chdmlm R. D. Evans, Wce Chairman UnlverOity of Chfcwo ~. In.Wttute of Technology Lewis slack,Secretary NstidFtem-cllmllntil E. c.Andmrmn HertwrtC?dd&tm i.mAlumnw. laboratory Calmibla Unlvertity C.J. BmkcwM J. J. Nlckmn M Ridge MU. Iabmntm-y NEmOrial Eo9pltd (New York) RobertG.cm- IL L. Phtzma!l A& MColbWaf TcmO A~ N81icad la.tamtory ugoFam D. M Vm P+ter NaUOadwruofsmldmda Eartol RO~ Fwdmon Oeorgew.Wetbefill Urdvarslty of CUlfonda (Ian Angeles) LIAISONMRMBERS padC. Aaberwld JeromeRegean AtOmlcEwrgy-mmfmtm UfIce ofNaml Reach Ja6ePh E. Duval J. &wwd McMlflen Afr Farce OfUce of SctenUflc ~h NuUc4ml Sdence FwndaUm SIJ9COMMlllEEON RADI-RMISTRY N. E. -, Chd- J. D. Kn@t u.& NavalFwlblO@dDOfenlm ICaAlamwSelmtlfic I.atmratmy Lab0rnk3m J. M. Nielmn G. R CbqPln 06netal Electric Company (IuChiand) FlOrMn State Uutvertiw G. D. O’ftdley E. M Clark W NMge National Laboratory Rmaselaer POlytadmlc fmtitute RP. tldmman R. M“ Dimnold AtOmlc mml’w rnvistml Lawrence Radiaklm Iabm-story Pfltlllpa Petroleum COi+my (LillM FalM) A.W.Fdrf@l K P. ~rg Unlversfty of Washl@m Argmne NatfOllaf mbodmy Jerome Ndls P. c.Wevanean Brcakkav671 Natfanal fdwratory Lawrence lwnatlan LabOrafov D. N. bhiernmn Mttelb Mernorhf fmlltute coNsLaTANl J. W. Ulncbetier “~wata hlefmuti of 1%~ ,. The Radiochemistry of Radium By H. W. Kirby Mound Laboratory Monsanto Research Corporation Miamisburg, Ohio and Murrell L. Salutsky W. R. Grace & Co. Research Division Washington Research Center Clarksville, Md. kued,December19&4 Subcommitteeon Radiochemistry NationalAcademy of Sciences—NaUonal Research Council Prin&dinUSA.Price$2.25Av-dhblefromtieClear4@cmseforFederal Sciendficu@ TechnknlInfonnatlon,Nation8.1BureauofStaxhr&,U.S.De- parbnentofComme-,SPri@ield,Vir@ia. -
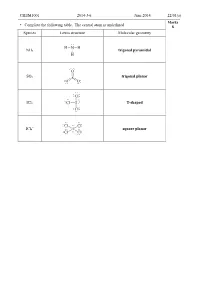
CHEM1001 2014-J-6 June 2014 22/01(A) • Complete the Following Table. the Central Atom Is Underlined. 8 Species Lewis Structu
CHEM1001 2014-J-6 June 2014 22/01(a) Marks • Complete the following table. The central atom is underlined. 8 Species Lewis structure Molecular geometry NH3 trigonal pyramidal SO3 trigonal planar ICl3 T-shaped – ICl4 square planar CHEM1001 2013-J-2 June 2013 22/01(a) Marks • Complete the following table, including resonance structures where appropriate. The 10 central atom is underlined. Is the species Species Lewis structure Molecular geometry polar? N F F F F NF3 Yes F N F Trigonal pyramidal S SO O S O O O Yes 2 V-shaped F F F F F F Cl ClF5 Yes Cl F F F F Square-based pyramidal H H B H BH3 B H No H H Trigonal planar THE REMAINDER OF THIS PAGE IS FOR ROUGH WORKING ONLY CHEM1001 2012-J-3 June 2012 22/01(a) Marks • Complete the following table, including resonance structures where appropriate. The 6 central atom is underlined. Is the molecule Species Lewis structure(s) polar? COCl2 yes CS2 no NBr3 yes SO2 yes • What is resonance? Give at least one example. 2 When two or more Lewis structures can be drawn for a molecule, the true structure is none of the structures that is drawn, but a type of average made up of all the resonance contributors. Some structures may contribute more than others. – For example, in NO3 , the ion does not contain 1 double and 2 single bonds, but is an average of the three structures shown. All of the N-O bonds are exactly the same length and the energy of the true structure is lower than the theoretical energy for any one of the given structures. -

Barium in Drinking-Water
WHO/SDE/WSH/03.04/76 English only Barium in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality © World Health Organization 2004 Requests for permission to reproduce or translate WHO publications - whether for sale of for non- commercial distribution - should be addressed to Publications (Fax: +41 22 791 4806; e-mail: [email protected]. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damage incurred as a results of its use. Preface One of the primary goals of WHO and its member states is that “all people, whatever their stage of development and their social and economic conditions, have the right to have access to an adequate supply of safe drinking water.” A major WHO function to achieve such goals is the responsibility “to propose ... regulations, and to make recommendations with respect to international health matters ....” The first WHO document dealing specifically with public drinking-water quality was published in 1958 as International Standards for Drinking-water. -

US5359094.Pdf
|||||||||||||I|| US005359094A United States Patent 19 11 Patent Number: 5,359,094 Teles et al. 45) Date of Patent: Oct. 25, 1994 (54) PREPARATION OF GLYCERYL 3,379,693 4/1968 Hostettler et al. .................. 549/229 CARBONATE 4,231,937 1/1980 Kao et al. ............ ... 549/229 4,314,945 2/1982 McMullen et al. ... 549/229 75 Inventors: Joaquim H. Teles, Ludwigshafen; 4,344,881 8/1982 Strege et al. ..... ... 549/229 Norbert Rieber, Mannheim; 4,483,994 11/1984 Jacobson ...... ... 549/229 Wolfgang Harder, Weinheim, all of 4,658,041 4/1987 Renga .......... ... 549/229 Fed. Rep. of Germany 4,835,289 5/1989 Brindapke ... ... 549/229 73) Assignee: BASF Aktiengesellschaft, 5,091,543 2/1992 Grey .................... ... 549/229 Ludwigshafen, Fed. Rep. of 5, 18,818, 6/1992 Delledonine et al. ............... 549/229 Germany FOREIGN PATENT DOCUMENTS (21) Appl. No.: 99,142 2222488 11/1972 Fed. Rep. of Germany. 2265228 12/1976 Fed. Rep. of Germany . 22 Filed: Jul. 29, 1993 1382313 1/1975 United Kingdom . 30 Foreign Application Priority Data Primary Examiner-Cecilia Tsang Aug. 5, 1992 (DE Fed. Rep. of Germany ....... 4225870 Attorney, Agent, or Firm-Keil & Weinkauf 51 Int. Cl. ............................................ CO7D 317/36 (57) ABSTRACT 52 U.S. C. .................................... 549/228; 549/229; 549/230 A process for the preparation of glyceryl carbonate by 58) Field of Search ........................ 549/228, 229, 230 causing glycerol to react with carbon monoxide and oxygen in the presence of a Group Ib, Group IIb, or (56) References Cited Group VIIIb catalyst as per the Periodic Table attem U.S. -

AP Chemistry Summer Assignment 2021
AP Chemistry Information and Summer assignment Overview of Course Expectations: AP Chemistry is a college level course. Students can earn up to 8 hours college credit for successful completion of this course and a good score on the AP Exam. It is a time-consuming and challenging, yet extremely rewarding, course. This course moves at a very fast pace and classroom attendance is a MUST. Students will be prepared to do college level work of any type upon completion of this course due to the thought processes used and the discipline/work habits required. The course covers two semesters of college chemistry. We will complete many hours of lab work. To have success on the AP exam, students will need to spend on average five to ten additional hours per week outside of class working on AP chemistry. This time will be spent on homework assignments, pre-labs, lab reports, problem sets, etc. There will be times that students will be able to complete things during class if they use their time efficiently. These statements are not meant to discourage, but to point out and state the truth to avoid any misconceptions about the high expectations for this course. I will do my very best to provide a college level course/experience which not only prepares you for the AP exam, but provides a solid foundation in chemistry. I also intend for it to be fun!! You are fortunate to be able to take this type of college level course in the high school setting as part of a small class.