UMKC Combined Chemicals List-Rcra P & U Listed, California Listedm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

(A) 1) at First a Pair of Each 10G. of Sample Was Extracted with Ether by the Soxhlet's Apparatus for a Week
52 [vol. 6, THE DISTRIBUTION OF PHOSPHORUS IN COW'S MILK AND THE SCHEME FOR THE SEPARATION OF PHOSPHATIDES By Rinjiro SASAKI (From the Institute of Agricultural Chemistry, College of Agriculture, Tokyo Imperial University) (Received 9th September 1930) A. The Distribution of Phosphorus in Cow's milk In order to extract the phosphatides, it is necessary first to determine the distribution of phosphorus in cow's milk. Liquid milk can not be extracted with ether or with other organic solvents owing to its large content of water. The substance applied in this experiment is the milk powder, dried by the Buflovak drum drier below 70•Ž. Its content of moisture is 4.498%, deter mined by the method of drying in the air bath of 105•Ž. The content of total phosphorus was determined(5) in the ash of sample which was burned with a little of fusing mixture. In 100g. of milk powder In 100g. of dry matter Total phosphorus 0.745g. 0.781g. The amounts of phosphorus, which are soluble in the various organic sol vents, were determined by extracting with three solvents in the following orders. (A) Ether-Acetone-Alcohol (B) Acetone-Ether-Alcohol (C) Alcohol-Ether-Acetone Experiment (A) 1) At first a pair of each 10g. of sample was extracted with ether by the Soxhlet's apparatus for a week. After the extraction had been comp leted, the extract was freed from ether and weighed. In 100g.of milkpowder In 100g.of dry matter Total ether matters soluble 3.609g. 3.779g. 2) The above extract was dissolved in a very little of ether. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Downloads/DL Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGI SCHE BEWERTUNGEN/Bewertungen/Toxbew072-L.Pdf
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: _____________________________ ______________ Jedidiah Samuel Snyder Date Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Master of Public Health Global Environmental Health _________________________________________ P. Barry Ryan, Ph.D. Committee Chair _________________________________________ Eugene Demchuk, Ph.D. Committee Member _________________________________________ Paige Tolbert, Ph.D. Committee Member Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Bachelor of Science in Engineering, B.S.E. The University of Iowa 2010 Thesis Committee Chair: P. Barry Ryan, Ph.D. An abstract of A thesis submitted to the Faculty of the Rollins School of Public Health of Emory University in partial fulfillment of the requirements for the degree of Master of Public Health in Global Environmental Health 2015 Abstract Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

United Nations LAWS and REGULATIONS FRANCE
E/NL.1950/9-16 1 March 1950 United Nations LAWS AND REGULATIONS PROMULGATED TO GIVE EFFECT TO THE PROVISIONS OF THE CONVENTION OF 13 JULY 1931 FOR LIMITING THE MANUFACTURE AND REGULATING THE DISTRIBUTION OF NARCOTIC DRUGS AS AMENDED BY THE PROTOCOL OF 11 DECEMBER 1946 FRANCE COMMUNICATED BY THE GOVERNMENT OF FRANCE Lake Success, New York, 1950 Note by the Secretary-General In accordance with Article 21 of the Convention of 13 July 1931 for Limiting the Manufacture and Regulating the Distribution of Narcotic Drugs, as amended by the Protocol of 11 December 1946, the Secretary-General has the honour to communicate here• after the texts of regulations. Ori gina1: English E/NL.1950/9 COMPOSITION OF PART II OF THE SCHEDULES OF POISONOUS SUBSTANCES* 1he Minister of Public Health and Population, •Considering the amended Act of 19 July 1845, Considering the Decree of 19 November 1943, and in particular Article 1 of the said Decree, Orders as follows: Article 1 The following substances shall be included in Schedule A, Part II: Arsenious oxide and arsenic acid Hydrocyanic acid Aconite (leaves, roots, extract and tincture) Aconitine and its salts Adrenaline Alkaloids of opium, their salts and their derivatives, other than those specifically mentioned in Schedule B Apomorphine and its salts Arecoline and its salts Arsenates and arsenites Metallic arsenic (cobalt) Atropine and its salts Belladonna (leaves, roots, powder and extract) Benzoate of mercury Dichloride of mercury Bi-iodide of mercury Bromo form Methyl bromide Brucine and its salts Cantharides (whole, powder and tincture) Cantharidin and its salts Chloroform Chloropicrin Hemlock (fruit, powder and extract) Codeine and its salts Colchicine and its salts Colchicum (seeds and extract) Conine and its salts Convalla toxin Indian berry The poisonous substances classified in the Codex without legal authority as "toxic" substances or as substances "to be kept separately" are not listed here. -

United States Patent Office Patented Apr
3,030,421 United States Patent Office Patented Apr. 17, 1962 2 3,030,421 perature and under a reduced pressure. In the case of PROCESS FOR PREPARNG TRHYDROXY most of the catalysts the reaction products, which are free METHYL-PHOSPHINE s from solvents, solidify already when being cooled to room Martin Reuter and Ludwig Ortane, Frankfurt an Main, temperature. If, in the case of some catalysts, they Germany, assignors to Farbwerke Hoechst Aktienger 5 solidify only at a lower temperature and still contain to sellschaft vormas Meister Lucius & Briining, Frank a greater extent oily by-products and/or phosphonium furt am Main, Germany, a corporation of Gerinally hydroxide, they can be separated from the latter by filter No Drawing. Fied Jaa. 14, 1958, Ser. No. 708,764 ing or pressing. - - - Claims priority, application Germany Jan. 23, 1957 It is to be assumed that the crystalline main product 6 Canas. (C. 260-606.5) O of the present invention constitutes the hitherto unknown We have found that a new and valuable phosphorus trihydroxymethyl-phosphine. Main and by-products are compound carrying hydroxymethyl groups at the phos easily soluble in water and methanol and sparingly solu phorus atom can be prepared by reacting 1 mol of formal ble in fat dissolvers. dehyde with 4 mol of phosphine, preferably in the pres The reaction products of the invention can be used as ence of water, and in the presence of Small quantities of 5 insecticides, additives for lubricants, flame-proofing agents finely distributed metals that do not belong to the alkali for wood and textiles and as intermediates for these sub metals or alkaline earth metals and/or their compounds Staces. -

Federal Law and Vertebrate Pest Control
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Proceedings of the 1st Vertebrate Pest Vertebrate Pest Conference Proceedings Conference (1962) collection February 1962 FEDERAL LAW AND VERTEBRATE PEST CONTROL Justus C. Ward Director, Pesticides Regulation Division, Agricultural Research Service, U.S. Department of Agriculture Follow this and additional works at: https://digitalcommons.unl.edu/vpcone Part of the Environmental Health and Protection Commons Ward, Justus C., "FEDERAL LAW AND VERTEBRATE PEST CONTROL" (1962). Proceedings of the 1st Vertebrate Pest Conference (1962). 25. https://digitalcommons.unl.edu/vpcone/25 This Article is brought to you for free and open access by the Vertebrate Pest Conference Proceedings collection at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Proceedings of the 1st Vertebrate Pest Conference (1962) by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. FEDERAL LAW AND VERTEBRATE PEST CONTROL By: Justus C. Ward, Director, Pesticides Regulation Division, Agricultural Research Service, U.S. Department of Agriculture Presented at the Vertebrate Pest Control Conference, Sacramento, California, February 6 and 7, 1 962 Shortly after the passage of the Federal Insecticide Act of 1910> mammal control specialists in the Bureau of Biological Survey began to consider a similar law to cover the chemicals with which they were concerned. Work on the project went slowly a nd spasmodically, but reached the point of having a Federal Rodenticide Act available for study and possible revision in 1928. At this time, the mammal control chemicals in use were limited to strychnine -- alkaloid and sulphate -arsenic, barium carbonate, th allium sulphate, phosphorus, s odium and calcium cyanide, carbon disulphide, and red squill. -
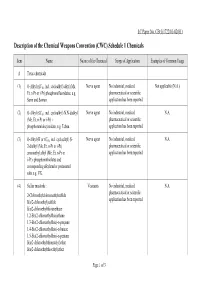
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

Introduetion
THE CONCENTFL4TION OF RADIUM AND MESO- THORIUM BY FRACTIONAL CRYSTALLIZATION* BY JOHN I,. NIERMAN Introduetion MarkwaldlO and Soddyl' have shown independently that mesothorium is absolutely identical in chemical nature with radium and cannot be separated therefrom.** In consequence all radium separated from uranium minerals containing thorium, contains also the mesothorium in the mineral, and all preparations of mesothorium contain the radium that is present in the mineral from which the thorium is derived. In the extraction and recovery of the minute quantities of mesothorium and radium present in radioactive minerals, these elements become associated with barium and follow the barium throughout the process. The refining of mesothorium and radium then consists in separating these elements from barium, the method generally followed being fractional crystallization of the barium solution, first as chloride, and later as bromide. The mesothorium and radium continue to be enriched in the crystal fractions, and reduced in the succes- sive mother liquors. t In practice, l2 a fair concentration of acid is maintained throughout the chloride and bromide systems, for the reason ' that the factor of enrichment of mesothorium radium chloride from barium chloride and also of mesothorium radium bromide from barium bromide is regarded as more favorable in acid than in neutral solutions. While it has been shown3 that the crystallization factor is higher for bromides than for chlorides, the effect of the acidity of the solutions on the progress of * Abstract of a thesis submitted in partial fulfilment of the requirements for the degree of Master of Arts in the Graduate, School of the University of Missouri, August, 1919. -

United States Patent (19) (11) 4,161,571 Yasui Et Al
United States Patent (19) (11) 4,161,571 Yasui et al. 45 Jul. 17, 1979 (54) PROCESS FOR PRODUCTION OF THE 4,080,493 3/1978 Yasui et al. .......................... 260/879 MALE CANHYDRDE ADDUCT OF A 4,082,817 4/1978 Imaizumi et al. ...................... 526/46 LIQUID POLYMER 4,091,198 5/1978 Smith ..................................... 526/56 75 Inventors: Seimei Yasui, Takarazuka; Takao FOREIGN PATENT DOCUMENTS Oshima, Sonehigashi, both of Japan 2262677 2/1975 France ....................................... 526/56 73) Assignee: Sumitomo Chemical Company, 44-1989 1/1969 Japan ......................................... 526/56 Limited, Osaka, Japan Primary Examiner-William F. Hamrock Attorney, Agent, or Firm-Birch, Stewart, Kolasch and 21 Appl. No.: 843,311 Birch 22 Filed: Oct. 18, 1977 57 ABSTRACT Related U.S. Application Data A process for production of the maleic anhydride ad duct of a liquid polymer having a maleic anhydride 62 Division of Ser. No. 733,914, Oct. 19, 1976, Pat, No. addition amount of 2 to 70% by weight, which com 4,080,493. prises reacting a liquid polymer having a molecular 51 Int. C.’................................................ CO8F 8/46 weight of 150 to 5,000 and a viscosity of 2 to 50,000 cp (52) U.S. C. ...................................... 526/90; 526/192; at 30 C. in the presence of at least one compound, as a 526/209; 526/213; 526/193; 526/195; 526/226; gelation inhibitor, selected from the group consisting of 526/233; 526/237; 526/238; 526/272; 525/285; imidazoles, thiazoles, metallic salts of mercapto 525/249; 525/251; 525/255; 525/245; 525/248 thiazoles, urea derivatives, naphthylamines, nitrosa (58) Field of Search ................ -

Effects of Intravenous Injections of Radium Bromide. by R
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central EFFECTS OF INTRAVENOUS INJECTIONS OF RADIUM BROMIDE. BY R. BURTON-OPITZ AND GUSTAVE M. MEYER. (From the Laboratories of Physiology and Physiological Chemistry of Colum- bia University, at the College of Physicians and Surgeons, New York.) PLATE XVI. The present study was undertaken with a view of determining in a general way the effects of intravenous administration of ra- dium upon the circulation and respiration. The problem was sug- gested to us by Dr. William J. Gies, under whose guidance a number of researches, dealing with the more extensive question of the action of radium upon animal and vegetable cells, have re- cently been carried on in the laboratories of Columbia University.1 For the radium used in these experiments we are greatly in- debted to Mr. Hugo Lieber. It was supplied to us in the form of the bromide, in preparations of 240 , iooo, and io,ooo activities. The strength of the solution used was the same in all cases. It contained 45 rag. of the dry substance in 25 c. c. of the solvent; each cubic centimeter of the solution, therefore, contained 1.8 rag. of the radium preparation. The amount of the radium present varies directly with the ra- dio-activity. Preparations of ~,5oo,ooo activity are said to repre- sent pure radium bromide3 Taking this figure as the standard of purity, ~ .8 rag. of the radium preparation of io,ooo activity contained approximately only o.o~ 26 mg., the same quantity of the preparation of ~ooo activity contained o.ooi26 rag.