Malignant Arrhythmic Mitral Valve Prolapse: a Continuum of Clinical Challenges from Diagnosis to Risk Stratification and Patient Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood Flow DHO8 7.8, Pg
Blood Flow DHO8 7.8, pg. 190 HS1/2017-2018 Circuits •Pulmonary circuit –The blood pathway between the right of the heart, to the lungs, and back to the left side of the heart. •Systemic circuit –The pathway between the left side of the heart, to the body, and back to the right side of the heart. The Pathway of Blood •Superior & Inferior Vena •Left Atrium Cava •Mitral Valve •Right Atrium •Left Ventricle •Tricuspid Valve •Aortic Semilunar Valve •Right Ventricle •Aorta •Pulmonary Semilunar -Arteries Valve -Arterioles •Pulmonary Artery -Capillaries •Lungs -Venules –Pulmonary Arterioles -Veins –Pulmonary Capillaries –Pulmonary Venules •Pulmonary Vein Blood Flow Through Heart Do You Know? • When blood leaves the left atrium, where does it go next? a) Aorta b) Left ventricle c) Right atrium d) Pulmonary artery And the answer is….A Do You Know? • After blood leaves the right atrium, what valve prevents the back flow? a) Pulmonary b) Mitral c) Tricuspid d) Aortic And the answer is…C Do You Know? • The right ventricle is the chamber of the heart that pumps blood for the pulmonary circulation. Based on this information, blood from the right ventricle is on its way to the _____. a) Liver b) Lungs c) Hands and feet And the answer is…B Do You Know? • Which of the following is correct order of blood flow for the right side of the heart? a) RA, Tricuspid valve, RV, PSLV, pulmonary artery b) RA, PSLV, RV, Tricuspid valve, pulmonary artery c) RA, Tricuspid valve, RV, pulmonary artery , PSLV And the answer is…A Do You Know? • Which of the following is correct order of blood flow for the left side of the heart? a) LA, Bicuspid valve, LV, ASLV, aorta b) LA, ASLV, LV, Bicuspid valve, aorta c) LA, Bicuspid valve, LV, ASLV, aorta And the answer is…C. -

Mitral Valve Prolapse
MITRAL VALVE PROLAPSE WHAT IS MITRAL VALVE PROLAPSE? The mitral valve is a heart valve with two tissue flaps, called leaflets, that open and close. It is located between the left upper chamber (atrium) and left lower chamber (ventricle) of the heart. Mitral valve prolapse occurs when the mitral valve bulges into the left atrium when the heart contracts (squeezes). This may keep the leaflets from closing properly. The result is a backward flow of blood into the left atrium. This is referred to as leaking or regurgitation. Most of the time, however, mitral valve prolapse causes no symptoms and no problems. WHAT ARE THE SYMPTOMS? Most people with mitral valve prolapse have no symptoms. However, some have brief periods of rapid heartbeat or skipped beats. Some people have sharp chest pains lasting seconds or minutes. Some people have shortness of breath when climbing stairs or with heavy exertion. You may notice symptoms more when you are physically active HOW IS IT DIAGNOSED? Often mitral valve prolapse is discovered during a physical examination, when the doctor listens to your heart with a stethoscope. The valve leaflets create a “click” sound that the doctor may hear. If the valve leaks, the doctor may also hear a murmur. The doctor may ask you to stand, sit, lie down, or squat during your exam, so he or she can better hear the heart sounds. An echocardiogram passes sound waves through the heart to create an image. It is the best test to diagnose mitral valve prolapse. The images show the prolapse, any thickening of the valve leaflets, and any leakage of blood through the prolapsed valve. -

Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: the Role of Multimodality Imaging to Detect High-Risk Features
diagnostics Review Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features Anna Giulia Pavon 1,2,*, Pierre Monney 1,2,3 and Juerg Schwitter 1,2,3 1 Cardiac MR Center (CRMC), Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland; [email protected] (P.M.); [email protected] (J.S.) 2 Cardiovascular Department, Division of Cardiology, Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland 3 Faculty of Biology and Medicine, University of Lausanne (UniL), 1100 Lausanne, Switzerland * Correspondence: [email protected]; Tel.: +41-775-566-983 Abstract: Mitral valve prolapse (MVP) was first described in the 1960s, and it is usually a benign condition. However, a subtype of patients are known to have a higher incidence of ventricular arrhythmias and sudden cardiac death, the so called “arrhythmic MVP.” In recent years, several studies have been published to identify the most important clinical features to distinguish the benign form from the potentially lethal one in order to personalize patient’s treatment and follow-up. In this review, we specifically focused on red flags for increased arrhythmic risk to whom the cardiologist must be aware of while performing a cardiovascular imaging evaluation in patients with MVP. Keywords: mitral valve prolapse; arrhythmias; cardiovascular magnetic resonance Citation: Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality 1. Mitral Valve and Arrhythmias: A Long Story Short Imaging to Detect High-Risk Features. In the recent years, the scientific community has begun to pay increasing attention Diagnostics 2021, 11, 683. -

Mitral Valve Prolapse Page 1 of 3
Mitral valve prolapse Page 1 of 3 Mitral valve prolapse Definition Mitral valve prolapse is a heart problem in which the valve that separates the upper and lower chambers of the left side of the heart does not close properly. Alternative Names Barlow syndrome; Floppy mitral valve; Myxomatous mitral valve; Billowing mitral valve; Systolic click-murmur syndrome; Prolapsing mitral leaflet syndrome Causes The mitral valve helps blood on the left side of the heart flow in one direction. It closes to keep blood from moving backwards when the heart beats (contracts). Mitral valve prolapse is the term used when the valve does not close properly. It can be caused by many different things. In most cases, it is harmless and patients usually do not know they have the problem. As much as 10% of the population has some minor, insignificant form of mitral valve prolapse, but it does not generally affect their lifestyle. In a small number of cases, the prolapse can cause blood to leak backwards. This is called mitral regurgitation. Mitral valves that are structurally abnormal can raise the risk for bacterial infection. Some forms of mitral valve prolapse seem to be passed down through families (inherited). Mitral valve prolapse has been associated with Graves disease . Mitral valve prolapse often affects thin women who may have minor chest wall deformities, scoliosis , or other disorders. Mitral valve prolapse is associated with some connective tissue disorders, especially Marfan syndrome . Other conditions include: • Ehlers-Danlos syndrome • Osteogenesis imperfecta • Polycystic kidney disease http://eclinicalworks.adam.com/content.aspx?ref=applications.adam.com&url=eclinicalwo .. -

Chapter 12 the Cardiovascular System: the Heart Pages
CHAPTER 12 THE CARDIOVASCULAR SYSTEM: THE HEART PAGES 388 - 411 LOCATION & GENERAL FEATURES OF THE HEART TWO CIRCUIT CIRCULATORY SYSTEM DIVISIONS OF THE HEART FOUR CHAMBERS Right Atrium Left Atrium Receives blood from Receives blood from the systemic circuit the pulmonary circuit FOUR CHAMBERS Right Ventricle Left Ventricle Ejects blood into the Ejects blood into the pulmonary circuit systemic circuit FOUR VALVES –ATRIOVENTRICULAR VALVES Right Atrioventricular Left Atrioventricular Valve (AV) Valve (AV) Tricuspid Valve Bicuspid Valve and Mitral Valve FOUR VALVES –SEMILUNAR VALVES Pulmonary valve Aortic Valve Guards entrance to Guards entrance to the pulmonary trunk the aorta FLOW OF BLOOD MAJOR VEINS AND ARTERIES AROUND THE HEART • Arteries carry blood AWAY from the heart • Veins allow blood to VISIT the heart MAJOR VEINS AND ARTERIES ON THE HEART Coronary Circulation – Supplies blood to the muscle tissue of the heart ARTERIES Elastic artery: Large, resilient vessels. pulmonary trunk and aorta Muscular artery: Medium-sized arteries. They distribute blood to skeletal muscles and internal organs. external carotid artery of the neck Arteriole: Smallest of arteries. Lead into capillaries VEINS Large veins: Largest of the veins. Superior and Inferior Vena Cava Medium-sized veins: Medium sized veins. Pulmonary veins Venules: the smallest type of vein. Lead into capillaries CAPILLARIES Exchange of molecules between blood and interstitial fluid. FLOW OF BLOOD THROUGH HEART TISSUES OF THE HEART THE HEART WALL Pericardium Outermost layer Serous membrane Myocardium Middle layer Thick muscle layer Endocardium Inner lining of pumping chambers Continuous with endothelium CARDIAC MUSCLE Depend on oxygen to obtain energy Abundant in mitochondria In contact with several other cardiac muscles Intercalated disks – interlocking membranes of adjacent cells Desmosomes Gap junctions CONNECTIVE TISSUE Wrap around each cardiac muscle cell and tie together adjacent cells. -

Heart Valve Disease: Mitral and Tricuspid Valves
Heart Valve Disease: Mitral and Tricuspid Valves Heart anatomy The heart has two sides, separated by an inner wall called the septum. The right side of the heart pumps blood to the lungs to pick up oxygen. The left side of the heart receives the oxygen- rich blood from the lungs and pumps it to the body. The heart has four chambers and four valves that regulate blood flow. The upper chambers are called the left and right atria, and the lower chambers are called the left and right ventricles. The mitral valve is located on the left side of the heart, between the left atrium and the left ventricle. This valve has two leaflets that allow blood to flow from the lungs to the heart. The tricuspid valve is located on the right side of the heart, between the right atrium and the right ventricle. This valve has three leaflets and its function is to Cardiac Surgery-MATRIx Program -1- prevent blood from leaking back into the right atrium. What is heart valve disease? In heart valve disease, one or more of the valves in your heart does not open or close properly. Heart valve problems may include: • Regurgitation (also called insufficiency)- In this condition, the valve leaflets don't close properly, causing blood to leak backward in your heart. • Stenosis- In valve stenosis, your valve leaflets become thick or stiff, and do not open wide enough. This reduces blood flow through the valve. Blausen.com staff-Own work, CC BY 3.0 Mitral valve disease The most common problems affecting the mitral valve are the inability for the valve to completely open (stenosis) or close (regurgitation). -
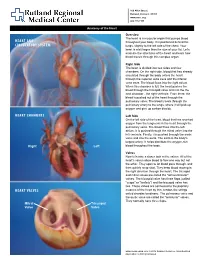
Heart and Circulatory System Heart Chambers
160 Allen Street Rutland, Vermont 05701 www.rrmc.org 802.775.7111 Anatomy of the Heart Overview The heart is a muscular organ that pumps blood HEART AND throughout your body. It is positioned behind the CIRCULATORY SYSTEM lungs, slightly to the left side of the chest. Your heart is a bit larger than the size of your fist. Let's examine the structures of the heart and learn how blood travels through this complex organ. Right Side The heart is divided into two sides and four chambers. On the right side, blood that has already circulated through the body enters the heart through the superior vena cava and the inferior vena cava. The blood flows into the right atrium. When this chamber is full, the heart pushes the blood through the tricuspid valve and into the the next chamber - the right ventricle. From there, the blood is pushed out of the heart through the pulmonary valve. The blood travels through the pulmonary artery to the lungs, where it will pick up oxygen and give up carbon dioxide. HEART CHAMBERS Left Side On the left side of the heart, blood that has received oxygen from the lungs enters the heart through the pulmonary veins. The blood flows into the left atrium. It is pushed through the mitral valve into the left ventricle. Finally, it is pushed through the aortic valve and into the aorta. The aorta is the body's largest artery. It helps distribute the oxygen-rich Right Left blood throughout the body. Valves Now let's take a closer look at the valves. -

Selecting Candidates for Transcatheter Mitral Valve Repair
• INNOVATIONS Key Points Selecting Candidates for • Mitral valve regurgitation, or leaky mitral valve, is a common valve disorder Transcatheter Mitral Valve Repair in which the leaflets of the mitral valve fail to seal effectively, resulting in Annapoorna S. Kini, MD Samin K. Sharma, MD some blood flowing back in the left atrium every time Mitral valve regurgitation is a common valve disorder The EVEREST (Endovascular Valve Edge-to- the left ventricle contracts. that causes blood to leak backward through the Edge Repair Study) II Trial was a randomized study This condition has been mitral valve and into the left atrium as the heart comparing the transcatheter approach using traditionally addressed muscle contracts. Mitral regurgitation can originate MitraClip®—a tiny cobalt chromium clip that sutures with open heart surgery. from degenerative or structural defects due to the anterior and posterior mitral valve leaflets—with aging, infection, or congenital anomalies. In contrast, surgery in patients with moderate to severe mitral • We use the latest imaging functional mitral regurgitation occurs when coronary regurgitation who are candidates for either procedure. techniques both to ensure artery disease or events such as a heart attack change that each patient is a good After five years, the study has demonstrated that the size and shape of the heart muscle, preventing candidate for the procedure, MitraClip was associated with a similar risk of death the mitral valve from opening and closing properly. In and to monitor their progress compared with mitral valve surgery after excluding people with moderate to severe mitral regurgitation, once the device is implanted. patients who required surgery within six months. -

Ventricular Anatomy for the Electrophysiologist (Part
Ventricular Anatomy for the REVIEW Electrophysiologist (Part II) SPECIAL 서울대학교 의과대학 병리학교실 서정욱 이화여자대학교 의학전문대학원 김문영 ABSTRACT The conduction fibers and Purkinje network of the ventricular myocardium have their peculiar location and immuno-histochemical characteristics. The bundle of His is located at the inferior border of the membranous septum, where the single trunk ramifies into the left and right bundle branches. The left bundle branches are clearly visible at the surface. The right bundles are hidden in the septal myocardium and it is not easy to recognize them. The cellular characters of the conduction bundles are modified myocardial cells with less cytoplasmic filaments. Myoglobin is expressed at the contractile part, whereas CD56 is expressed at the intercalated disc. A fine meshwork of synaptophysin positive processes is noted particularly at the nodal tissue. C-kit positive cells are scattered, but their role is not well understood. Purkinje cells are a peripheral continuation of bundles seen at the immediate subendocardium of the left ventricle. Key words: ■ conduction system ■ Purkinje network ■ pathology ■ arrhythmia ■ electrophysiology Introduction human heart. In this brief review, the histological characteristics of conduction cells, stained by The functional assessment of abnormal cardiac conventional and immuno-histochemical staining, are 3 rhythm and a targeted treatment based on demonstrated in the second part of the review. electrophysiologic studies are successful advances in cardiology.1 Morphological assessment or confirmation The characteristic location of the ventricular of hearts with such abnormalities is rare, not only due conduction system to the limited availability of human hearts but also inherent technological limitations of existing The atrioventricular node is situated in its technology.2 Classical morphological approaches and subendocardial location at the triangle of Koch. -

Mitral Valve Surgery with the Edwards Pericardial Mitral Bioprosthesis
Mitral Valve Surgery With the Edwards Pericardial Mitral Bioprosthesis What You and Your Loved Ones Should Know Introduction This guide is for patients who have mitral heart valve disease and whose doctors have proposed surgery to replace the valve. It will help you and your loved ones learn more about your heart and how it works. You will also learn about valve disease and surgery options. Be sure to ask your doctor to explain the treatment choices and the heart valves used for surgery. This booklet does not include everything you need to know about heart valves, heart valve replacement surgery, or about related medical care. Regular check-ups by your heart doctor are important. Call or see your doctor whenever you have questions or concerns about your health, especially if you have any unusual symptoms or changes in your overall health. Table of Contents How does your heart work?....................................................................................................................XX What is mitral valve disease?..................................................................................................................XX How is mitral valve disease treated?.......................................................................................................XX What are your treatment options?..........................................................................................................XX What are your surgical mitral valve options?..........................................................................................XX -

Mitral Valve Prolapse: Definition and Implications in Athletes
JACC Vol. 7. No I 231 January 1986:231-6 Mitral Valve Prolapse: Definition and Implications in Athletes ROBERT M. JERESATY, MD, FACC Hartford and Farmington. Connecticut Mitral valve prolapse is probably the most common car• and sudden death. The symptoms and the complications diac valve disorder, affecting approximately 5 % of the are not usually related to physical activity. population. Although it is genetically determined, its A permissive attitude toward participation of patients clinical manifestations do not usually become evident with mitral valve prolapse in competitive athletics is before adulthood. In the setting of a cardiology referral probably warranted; however, it would appear reason• center, a mitral valve prolapse syndrome, consisting of able to disqualify athletes with mitral valve prolapse in nonspecific symptoms, repolarization changes on the the following circumstances: 1) history of syncope; 2) electrocardiogram and arrhythmias, has been identified. disabling chest pain; 3) complex ventricular arrhyth• However, doubt has recently been expressed about the mias, particularly if induced or worsened by exercise; existence of such a syndrome. The prognosis of mitral 4) significant mitral regurgitation; 5) prolonged QT in• valve prolapse is generally favorable but infrequent com• terval; 6) Marfan's syndrome; and 7) family history of plications do occur and include transient ischemic at• sudden death. tacks, progression of mitral regurgitation with or with• (J Am Coil CardioI1986;7:231-6) out ruptured chordae tendineae, -

Heart – Inflammation
Heart – Inflammation 1 Heart – Inflammation Figure Legend: Figure 1 Heart, Valve - Inflammation, Acute in a female F344/N rat from a chronic study. Neutrophils (arrows) are present within a valve. Figure 2 Heart, Valve - Inflammation, Suppurative in a female F344/N rat from a chronic study. Suppurative inflammation is present in the mitral valve (arrows). Figure 3 Heart, Valve - Inflammation, Suppurative in a female F344/N rat from a chronic study (higher magnification of Figure 2). Suppurative inflammation of the mitral valve has aggregates of bacteria (arrows). Figure 4 Heart, Valve - Inflammation, Chronic in a female F344/N rat from a chronic study. Mixed inflammatory cells are present within the thickened mitral valve (arrows). Figure 5 Heart, Valve - Inflammation, Chronic in a female F344/N rat from a chronic study (higher magnification of Figure 4). Mixed inflammatory cells and proliferation of mesenchymal cells result in thickening of the mitral valve. Figure 6 Heart, Valve - Inflammation, Chronic in a female F344/N rat from a chronic study (higher magnification of Figure 4). Lymphocytes, macrophages, and plasma cells with proliferating mesenchymal cells are present in the mitral valve. Figure 7 Heart - Inflammation, Chronic-active in a male F2 generation CD1 mouse. Inflammation of the heart along the endocardium consists primarily of neutrophils and macrophages. Figure 8 Heart - Inflammation, Chronic-active in a male B6C3F1/N mouse from a chronic study. Chronic active inflammation is present along the epicardial surface of the heart. Comment: Inflammation in the heart may occur in the myocardium, endocardium, or epicardium or may be associated with the valves.